
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Solve for the missing properties in the
off your final answers to SIX decimal places and summarize in one table at the end part of your solution to part I.
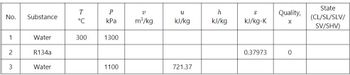
Transcribed Image Text:No.
1
2
3
Substance
Water
R134a
Water
T
°C
300
P
kPa
1300
1100
V
m³/kg
U
kJ/kg
721.37
h
kJ/kg
S
kJ/kg-K
0.37973
Quality,
X
0
State
(CL/SL/SLV/
SV/SHV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
show your solution at number 3
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
show your solution at number 3
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Thermodynamics Question: Can you please reduce the first law energy balance for: Ideal gas is compressed in a cylinder piston isothermally. No input or output streams. No change in potential or kinetic energy. Please write the first law, show what terms are zero and simplify it. Thank you.arrow_forwardDerive Newton's Raphson Formula from scratch using Taylor seriesarrow_forwardSolve the following problems SYSTEMATICALLY. Show your complete solution and enclose your final answers in a box. Steam with a specific volume of 0.09596 m/kg undergoes a constant pressure process at 1.70-MPaa until the specific volume becomes 0.13796 m³/kg. What are the (a) the final temperature, (b) Au, (c) W, (d) As and (e) Q?arrow_forward
- SolVe correctly please. There are 500 liquid medicines, 100 grams each, in a box. In how many minutes can a refrigerator with a power of 0.45 kW and an efficiency coefficient of 2.5 cool the medicines from 20 ° C to 8 ° C? The specific heat of the drug is given as 4.2 kJ/kg °C.arrow_forwardIf the mass of the string and the cup are so small that they are negligible, what is the approximate minimum number of 10-cent coins needed to break a bar of chocolate that has a flexural stress of 515,000 Pa? The length, thickness, and width measurements of the chocolate bar are 12mm each, and the mass of one 10-cent coin is 2.5g. Answer : zero, one, two or five?arrow_forwardMATCH THE ANSWER WITH THE QUESTION Path Function Choose. Choose. Isobaric Process Pressure is constant Нeat Intensive property No work done Pressure Adiabatic Process Volume No transfer of heatarrow_forward
- Show solutionarrow_forwardCompletely solve and box the final answer. Write legibly 4. Compute the heat conducted in KW through a sheet of plate glass, k = 0.025 Cal/s-cm-ºC, which is 3.5 m x 4.8 m and 12.5mm thick. The temperature of the surfaces are 28ºC and negative 5.4ºC.arrow_forwardThe pressure increase of a gas was divided into three calculation paths; Arrange the temperature of each path in increasing magnitude: Isothermal compression(T1) isochoric heating(T2 adiabatic compression(T3). a.T2<T1<T3 b.T1<T2<T3 c.T1<T3<T2 d.T2<T3<T1arrow_forward
- The specific heat of copper is 0.093 cal/(g.°C) and the specific heat of gold is 0.031 cal/(g.°C). If 5.8 cal is supplied to one gram of copper and one gram of gold, the RATIO of temperature increase of gold to that of copper isarrow_forwardSolve it correctly please. I will rate accordingly with 4votes.arrow_forwardnumber 1 A food product containing 82% moisture content is being frozen. Estimate the specific heat of the product at -8 ° C when 82% of the water is frozen. The specific heat of the dry product is 2.5 kJ / (kg ° C). It is assumed that the specific heat of water at -10 ° C is the same as the specific heat of water at 0 ° C, and that the specific heat of ice follows the function Cp es = 0.0062 T Frozen + 2.0649. Cp of frozen product = kJ / kg ° C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY