
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Do the equilibria of the following acid–base reactions lie to the right or the left? (The pKa of H2O2 is 11.6.)
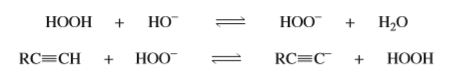
Transcribed Image Text:НО
НОО
НООН
Н.о
НОО
RC=CH
RC-C + НООН
Expert Solution
arrow_forward
Step 1
A reaction is said to be in equilibrium if the rate of the forward reaction is equal to the rate of the backward reaction.
arrow_forward
Step 2
The value of pKa is inversely proportional to the strength of an acid. Weaker acid has a high value of pKa. The equilibrium favors the formation of the weak acid.
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the approximate pK, of the following and indicate the most acidic hydroge (a) CH3-NH-CH3 (b) CH3-C=CH (c) CH3-COOH (d) CH3-CH2-OHarrow_forward12. Bicine is an organic compound used as a buffering agent. It is one of Good's buffers and has a pKa of 8.354 at 25 °C. The molecular formula of bicine is C6H13NO4. Bicine can react with Ba(OH)2 according to the following reaction. 2 C6H13NO4 + Ba(OH)2 → Ba(C6H12NO4)2 + 2 H₂O It took a student worker 3 steps to make a buffer solution. First, a bicine solution is made by dissolving 37.47 grams bicine in enough water. The total volume is 200.00 mL. Then 250.00 mL Ba(OH)2 solution is prepared by adding 36.57 grams Ba(OH)2 solid and enough water. At the end, a buffer solution is made by mixing 157.89 mL of the above bicine solution and 89.32 mL of the above Ba(OH)2 solution. A. B. C. Determine the equilibrium concentration of bicine in the buffer solution. Please provider your answer below. A of Ba(C6H12NO4)2 in the buffer solution. Check answer Determine the equilibrium concentration M Please provider your answer below. A pH = Please provider your answer below. A $ Check answer…arrow_forwardConsider the following equilibrium: O || R-C-H + HCN HO OH R-C-H | CN (A) When R = CH3CH2-, Keq = 1. (i) Predict whether Keq should be greater or less than 1 when R = CICH2, and (ii) when R is CH2=CH. Explain. (B) In the case where R is CH2=CH, the cyanohydrin is formed faster but given enough time, another constitutional isomeric C4H5NO product predominates. Explain and write a base-catalyzed mechanism for the formation of the other isomer. Recall that: HO HON Narrow_forward
- Which is the oxidant in the reactant side for each of the following reactions. Use the change of its oxidation numbers from the reactant to the product to identify it. (a) KH + H2O ---> KOH + H2 (b) 2 KClO3 + 3 C ---> 2 KCl + 3 CO2(g)arrow_forwardPlease answer these questionsarrow_forward(Q12) Hydrazoic acid (HN3) is a weak acid with a Ką of 2.5 x 105. What will be the equilibrium pH of a solution that is initially 0.349 M in hydrazoic acid?arrow_forward
- 15.44 Identify the following glycerophospholipid, which helps con- duct nerve impulses in the body, as a lecithin or cephalin, and list its components: O || CH,−O−C—(CH)4—CH, O || CH–0–C− (CH2)16—CH3 CH,−0-P-0–CH2–CH,-N–CH, O || Albo CH3 4+ CH3arrow_forwardGiven the following compounds and boiling points: Determine the IMFs Order the compounds from weakest to strongest IMFs, and Pentane (C5H12) 36°C Methane (CH4) -164°C Sodium n-butoxide (C4H9NaO) +260°C Hydrogen dioxide (H2O) 100°Carrow_forwardChoose the correct relationship between Kc and Kpfor:2 NF3(g)N2(g) + 3 F2(g) Kp = Kc(RT)-2 Kp = Kc(RT)1/2 Kp= Kc-2 Kp = Kc(RT)2arrow_forward
- Duestion 4 actirity of Lo mg of 11(t y2 = 1.7 y07 years) What is the of 1.0arrow_forwardCarbonic anhydrase is one of the fastest enzymes known. If 10 µg of this enzyme catalyzes the conversion of 0.30 g of CO2 to bicarbonate in 1 minute at 37˚C. What is the Kcat value for this enzyme in min -1?arrow_forward2. Consider the reaction scheme below: Bu;SnH 6-endo-trig 5-exo-trig Bu,SnH k = 4 x 10° s1 k= 2 x 105 s1 2% 98% Why does the 5-exo-trig pathway dominate? Provide a suitable diagram to explain your answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY