
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
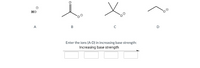
Transcribed Image Text:но
A
D
Enter the ions (A-D) in increasing base strength:
Increasing base strength
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How would I the conjugate acid and the base including ions pair and electronarrow_forwardThe pH of an aqueous solution at 25°C was found to be 3.40. ail The pOH of this solution is The hydronium ion concentration is M. The hydroxide ion concentration is M.arrow_forwardLabel (i) the strongest acid and (ii) the weakest acid. COOH COOH COOH CI .COOH ČI Br Br (A) (B) (C) (D) (i) Strongest acid- (ii) Weakest acid -arrow_forward
- Select the strongest acid in aqueous solution. These acids all have the same strength in aqueous solutions. НOBr HOI HOCIarrow_forwardMolecules that are amphoteric are not capable of reacting with both acids and bases - true or falsearrow_forwardThe Bronsted-Lowry (B-L) definition of acids and bases was developed mainly because there were problems with the Arrhenius definition that kept some acids and/or bases from being classified properly. Which of the following explains the problem of the Arrhenius definition and how the Bronsted-Lowry definition help fix the classification problems. The Arrhenius definition only classified acids/bases dissolved in water as well as bases were only those that released hydroxide ions into solution. Where in the B-L definition the acid & base does not have to be in a water solution and it says a base is any substance that accepts a hydrogen ion. The Arrhenius definition only classified acids/bases dissolved in water as well as bases were only those that released hydrogen ions into solution. Where in the B-L definition the acid & base does not have to be in a water solution and it says a base is any substance that accepts a hydrogen ion. The Arrhenius definition only classified acids/bases…arrow_forward
- In the context of acid-base chemistry, what is the function of a Bronsted-Lowry base? A) To donate a proton (H⁺)B) To accept a proton (H⁺)C) To donate a pair of electronsD) To accept a pair of electronsarrow_forwardThe pH of a 0.30 M solution of a weak base B is 9.02. What is the K, of the base? Be sure your answer has the correct number of significant digits. Kb X Sarrow_forwardThe pH of a solution of 0.50 M NH4Cl isarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY