
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
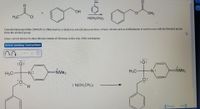
Transcribed Image Text:NMO2
ОН
CH3
H3C
CI
N(CH2CH3)3
Dimethylaminopyridine (DMAP) is often used as a catalyst in esterification reactions. A basic solvent such as triethylamine is used to react with the liberated proton
from the alcohol group.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
:ö:
:0:
:O:
:N-
NME2
H3C-
:N-
NME2
H3C
H.
: N(CH,CH3)3
Previous
Next
O:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the reagents necessary for the following hydrolysis. OMe NH3 ROH, pyridine O [H+], MeOH O H3O+ ? CI. CI OH + MeOHarrow_forwardShow steps and reagents necessary to obtain the following products in reactions. (Ex: process could be addition of bromine, hydrogenation, hydroboration, addition of HOX, elimination reactions, oxymercuration/demurcuration, etc) 4) Br H H. Br 5) H. H H 6) онarrow_forwardProvide a mechanism for the following reaction. Show each step in your mechanism and use curved arrows to show the movement of electron pairsarrow_forward
- Give the organic product formed in each of the following reactions, draw a 3-D representation for the product molecule showing the correct configurationarrow_forwardas content is proocted and Provide the reagents necessary to complete the following reactions. More than one step may be necessary, if so number separate steps. LOH -OH OH OH + en CHEM 2251arrow_forwardOrganic chemistry quarrow_forward
- =O =O Q3. Propose five methods to prepare the benzoic acid using five different starting materialsarrow_forwardWhich of the following reactions will not generate an alcohol group in the product? (1) CH,CH;MgBr Reaction A (2) dil. H* /H;O NaOH Br Reaction B H2O//THF (1) Hg(OAc)/ H0 Reaction C (2) NABH/ethanol NAOH Reaction D H2O//THF OReaction B Reaction A Reaction C Reaction Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY