
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
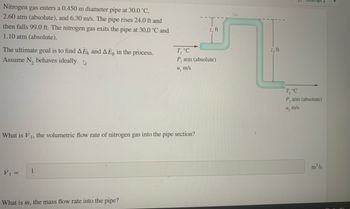
Transcribed Image Text:Nitrogen gas enters a 0.450 m diameter pipe at 30.0 °C,
2.60 atm (absolute), and 6.30 m/s. The pipe rises 24.0 ft and
then falls 99.0 ft. The nitrogen gas exits the pipe at 30.0 °C and
1.10 atm (absolute).
The ultimate goal is to find AEK and AEp in the process.
Assume N₂ behaves ideally.
What is V₁, the volumetric flow rate of nitrogen gas into the pipe section?
V₁ =
1
What is m, the mass flow rate into the pipe?
z, ft
T, °C
P, atm (absolute)
u, m/s
z, ft
T₂ °C
P, atm (absolute)
u, m/s
m³/s
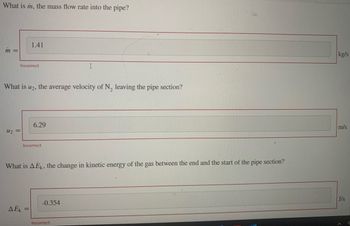
Transcribed Image Text:What is m, the mass flow rate into the pipe?
m =
Incorrect
U₂ =
1.41
What is U2, the average velocity of N₂ leaving the pipe section?
Incorrect
AEK =
6.29
=
What is AEK, the change in kinetic energy of the gas between the end and the start of the pipe section?
I
-0.354
Incorrect
kg/s
m/s
J/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 10 images

Knowledge Booster
Similar questions
- Fluid Mechanics Quiz 5 Q1: Air at 200 °F and 60 psia flows in a 4-in.-diameter pipe at a rate of 0.52 lb/s. Determine the pressure at the 2-in.-diameter throat of a Venturi meter placed in the pipe. Q2: Water flows through a 40-mm-diameter nozzle meter in a 75-mm-diameter pipe at a rate of 0.015 m/s. Determine the pressure difference across the nozzle if the temperature is (3) 10 °C. or (b) 80 °c. Q3: Water flows through the orifice meter shown in Fig. below at a rate of 0. 10 cfs. If d = 0.1 ft, determine the value of h. Q- 2 in.arrow_forwardSaturated liquid ammonia is entering a 50 cm diameter 10-m long pipe at a steady 0.1 kg/s mass flow rate and a mean temperature of 0°C. It is exiting the pipe as saturated vapor at a mean temperature of 0°C. (a) What is the Reynolds number and flow regime at the beginning and at the end of the pipe? (b) If the surface temperature of the pipe can be assumed to be constant at 15°C in this section, what is the average convection coefficient of the ammonia flow? (a) 1343; 27075 (b) 553.6 W/m2Karrow_forwardAn air pipe carries cool air at an inlet bulk temperature of 15C and a velocity of 12 m/s. The pipe is made of carbon steel of 0.25 m diameter, it is not insulated, and it( the pipe) will be maintained at temperature of 120C in order to raise the flowing air temperature to 45C. Calculate the Reynolds number, the heat transfer coefficient in W/(m^2K) and the lenght of this pipe in meters. Use Table A.4 to read the air properties.arrow_forward
- A ventilation opening in a room is 2 feet by 3 feet. Air flow out of the opening is measured at 75 feet per minute. Calculate the CFM rate.arrow_forward2. A natural gas is flowing through a 1 m diameter and 6.5 km long pipeline (friction factor= 0.0024) at a constant temperature of 15 °C. The pressure at the inlet of the pipe is 5.7 MPa. What is the maximum mass flow rate of the gas that can be achieved and the corresponding downstream pressure? [Ans. Gmax = 1012.3 kg s-¹, Pw = 498.6 kPa]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The