
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
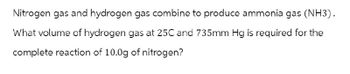
Transcribed Image Text:Nitrogen gas and hydrogen gas combine to produce ammonia gas (NH3).
What volume of hydrogen gas at 25C and 735mm Hg is required for the
complete reaction of 10.0g of nitrogen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- How many milliters of O2 would be produced at 18.2ºC and 749.4 mmHg by the reaction of 1.398 g of Na2O2 with an excess of CO2 present?arrow_forwardThe Haber Process synthesizes ammonia at elevated temperatures and pressures. Suppose you combine 1580 L of nitrogen gas and 4590 L of hydrogen gas at STP, heat the mixture to run the reaction, then isolate the ammonia from the reaction mixture. What volume of NH₃ , measured at STP, would be produced? Assume the reaction goes to completion. N₂ (g) + 3 H₂ (g) → 2 NH₃ (g)arrow_forwardIf 620 g of ZnS is to be reacted, what volume of oxygen (O2) at 0.877 atm and 34.0°C is needed to carry out this reaction?arrow_forward
- Ammonia gas which is NH3(g) is produced from the reaction of nitrogen gas which is N2(g) what volume of ammonia in KL can be obtained from the complete reaction of 7.5 kg of hydrogen at a pressure of 450 kPa and a temperature of 80 c?arrow_forwardWhat volume (in mL, at 373 K and 5.34 atm) of oxygen gas is required to react with 6.69 g of Al?arrow_forwardThe Haber Process synthesizes ammonia at elevated temperatures and pressures. Suppose you combine 1580 L of nitrogen gas and 4240 L of hydrogen gas at STP, heat the mixture to run the reaction, then separate the ammonia from the reaction mixture. What volume of reactant, measured at STP, is left over? Assume the reaction goes to completion. N₂ (g) + 3 H₂ (g) → 2 NH₃ (g)arrow_forward
- Roasting galena [lead(II) sulfide] is an early step in the industrial isolation of lead. How many liters of sulfur dioxide, measured at STP, are produced by the reaction of 6.45 kg of galena with 145. L of oxygen gas at 220 °C and 2.00 atm? Lead(II) oxide also forms. Be sure your answer has the correct number of significant figures. Note: Reference the Fundamental constants table for additional information. L SO₂ x10 Xarrow_forwardWhat volume of hydrogen gas is formed at 30oC, and 950 mmHg, when 5.00g of calcium reacts with 250.0 mL of 0.700M hydrochloric acid.arrow_forwardWhat would be the pressure of H2 (g) formed by the reaction of 89 grams of Al performed in a 10L flask at 85 degrees celsius?arrow_forward
- What volume of O2 at 988 mmHg and 31 °C is required to synthesize 23.5 mol of NO?arrow_forwardIn an experiment, 175 mL of nitrogen gas was collected over water at 23.5 oC and 757 mmHg. What mass of nitrogen gas was obtained? At the temperature of the gas (23.5 oC), the vapor pressure of water is 21.8 mmHg.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY