
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
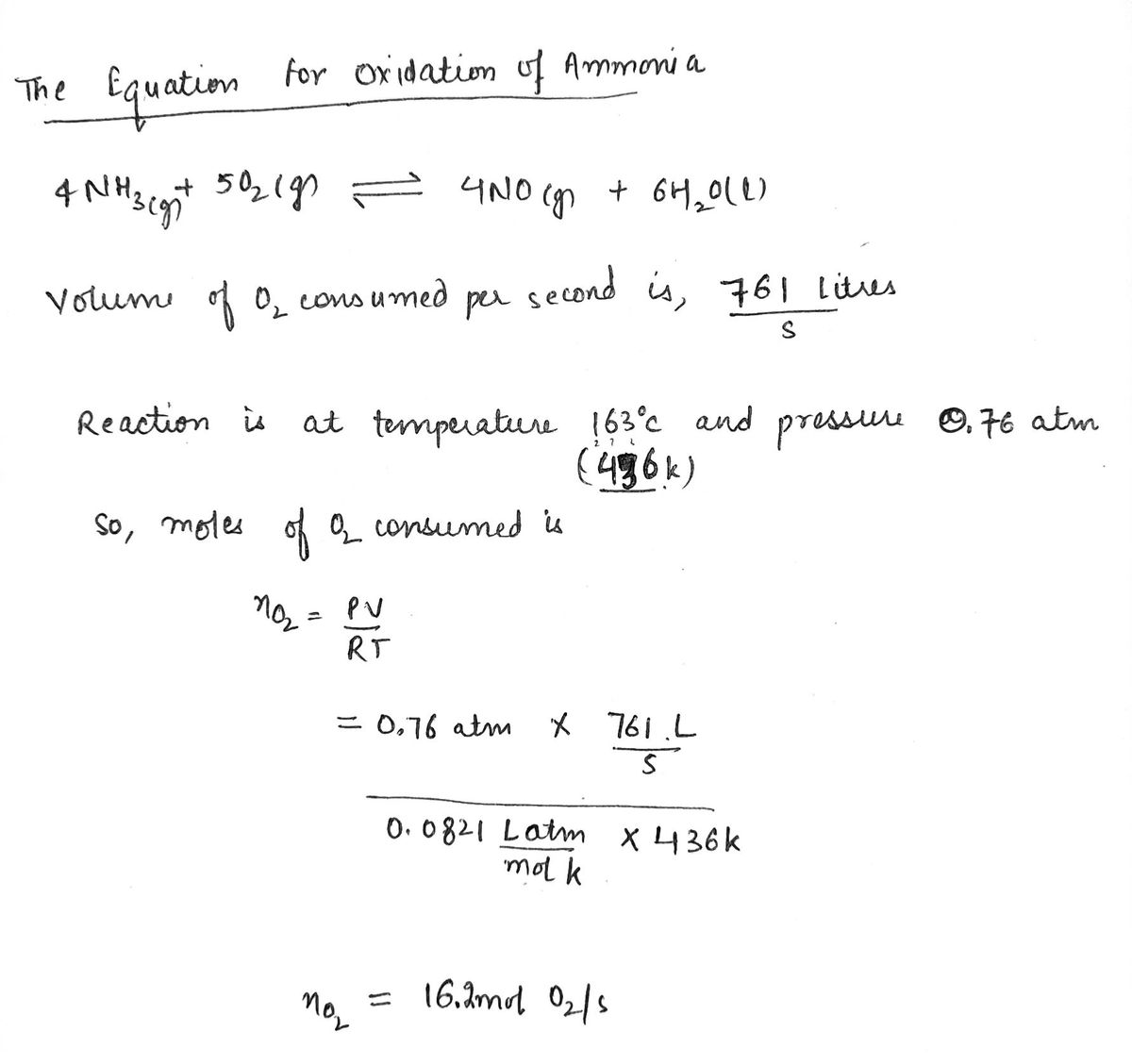
Transcribed Image Text:Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In
this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water.
Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 761. liters per second of dioxygen are
consumed when the reaction is run at 163. °C and the dioxygen is supplied at 0.76 atm. Calculate the rate at which nitrogen monoxide is being
produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits.
kg
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Most of the sulfur used in the United States is chemically synthesized from hydrogen sulfide gas recovered from natural gas wells. In the first step of this synthesis, called the Claus process, hydrogen sulfide gas is reacted with dioxygen gas to produce gaseous sulfur dioxide and water. Suppose a chemical engineer studying a new catalyst for the Claus reaction finds that 976 liters per second of dioxygen are consumed when the reaction is run at 269°C and the dioxygen is supplied at .060 atm. Calculate the rate at which sulfur dioxide is being produced. Give your answer in kilograms per second. Round your answer to significant digits.arrow_forwardNitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 299. liters per second of dioxygen are consumed when the reaction is run at 183. °℃ and the dioxygen is supplied at 0.39 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits.arrow_forwardNitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 573. liters per second of dioxygen are consumed when the reaction is run at 290. °C and the dioxygen is supplied at 0.77 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. kg Ox10 ?arrow_forward
- Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 150. liters per second of dioxygen are consumed when the reaction is run at 195. °C and the dioxygen is supplied at 0.22 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. kg x10 Sarrow_forwardNitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 985. liters per second of dioxygen are consumed when the reaction is run at 241. °C and the dioxygen is supplied at 0.29 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits.arrow_forwardIn the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 715. liters per second of dinitrogen are consumed when the reaction is run at 153. °C and the dinitrogen is supplied at 0.18 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. kg S Continue dallall 80 x10 X 802 S ? JUN 27 © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | A O Submit Assi tv .arrow_forward
- In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 122. liters per second of dinitrogen are consumed when the reaction is run at 179. °C and the dinitrogen is supplied at 0.59 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. 0 kg V 0 x10 Xarrow_forwardMost of the sulfur used in the United States is chemically synthesized from hydrogen sulfide gas recovered from natural gas wells. In the first step of this synthesis, called the Claus process, hydrogen sulfide gas is reacted with dioxygen gas to produce gaseous sulfur dioxide and water. Suppose a chemical engineer studying a new catalyst for the Claus reaction finds that 592. liters per second of dioxygen are consumed when the reaction is run at 195. °℃ and the dioxygen is supplied at 0.48 atm. Calculate the rate at which sulfur dioxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits.arrow_forwardMost of the sulfur used in the United States is chemically synthesized from hydrogen sulfide gas recovered from natural gas wells. In the first step of this synthesis, called the Claus process, hydrogen sulfide gas is reacted with dioxygen gas to produce gaseous sulfur dioxide and water. Suppose a chemical engineer studying a new catalyst for the Claus reaction finds that 421. liters per second of dioxygen are consumed when the reaction is run at 280. °C and the dioxygen is supplied at 0.35 atm. Calculate the rate at which sulfur dioxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits. 00 S X 6 000 Ar Barrow_forward
- Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 594. liters per second of dioxygen are consumed when the reaction is run at 276. °℃ and the dioxygen is supplied at 0.51 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits. kg S × Śarrow_forwardNitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 978. liters per second of dioxygen are consumed when the reaction is run at 272. °℃ and the dioxygen is supplied at 0.91 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits. ៣ kg S x10 Xarrow_forwardric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 946. liters per second of dioxygen are consumed when the reaction is run at 175. °C and the dioxygen is supplied at 0.73 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits. kg VA X olo Arlarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY