
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Would you expect the given compund to be more soluble in 0.1M strong acid or 0.1M strong base solution. Justify by indicating the appropriate
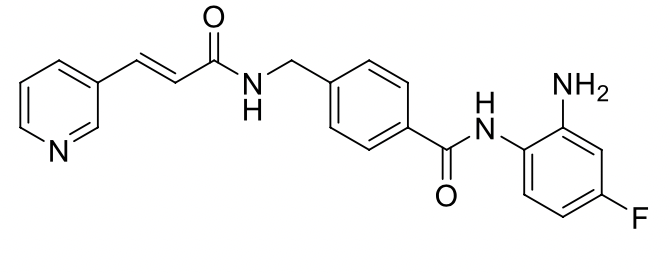
Transcribed Image Text:NH2
'F
エZ
Zエ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q.4. Circle the compound with the highest solubility in water and cross out the compound with the lowest solubility in water. CI OH CI OH Cl Brarrow_forwardA student dissolved 4 drops of an unknown compound in 1 mL of methylene chloride (dichloromethane) in a test tube. They added 5 drops of a 2% solution of bromine in dichloromethane dropwise and mixed the contents within the test tube gently. They observed the red-brown color discharges (turns colorless) until the last drop. What compound is most likely the unknown? ©GMU 2020 Oa. Methanol b. Cyclohexane c. n-Butyl Bromide O d. Hex-2-ene COLE QUESTION 6 A student placed 2 drops of an unknown sample in a test tube and added 2 mL of ethanol to the test tube while mixing gently. They added 2 drops of potassium permanganate reagent to the test tube and mixed the contents of the test tube gently. The initial color was a deep purple but then changed to a yellow color which precipitates as a brown solid. What compound is most likely the unknown? OGMU 2020 a. Cyclopentane O b. Cyclohexene c. 1-Pentyl Chloride d. 2-Propanol SOUL QUESTION 7 A student performed the Beilstein test within a chemical…arrow_forwardQ9 9a: Rank the water solubility of the following compounds: H2N NH2 CH3-0-CH2CH3 H3C. H3C° NH2 TH. 9b: Certain C-H functional groups can form weak hydrogen bonds. Why would such a functional group be more likely to be a H-bond donor when the C (carbon) is next to N (nitrogen)? (explain in 2-3 sentences) 9c: Explain why water forms nearly spherical droplets on the surface of a freshly waxed car. Define the effect from your knowledge in biochemistry (explain in 2-3 sentences)arrow_forward
- What would be the theoretical and % yield ?arrow_forwardPlease help solve this problemarrow_forwardJustify the solubility of the samples in the corresponding solvent based on their structure. Sample water 5% NaHCO3 5% HCl 5%NaOH Conc. H2SO4 ether Aniline n-butyl amine Acetic acid Phenol cyclohexanol 2-Propanol Benzoic acid bromobenzene Ethylene glycolarrow_forward
- My results do follow the statement that KHT has a low solubility at room temperature that generally increases with temperature.arrow_forwardYou may have observed that KHT has a low solubility at room temperature that generally increases with temperature. Based on your results, is the low solubility of KHT at room temperature a result of an unfavorable AH° or an unfavorable AS° value. Give your reasoning.arrow_forwardQuestion 17 Choose the situation below that would result in an exothermic AHsolution- When |AHsolventl JAHsolutel >> When JAHjatticel > JA Hnydrationl When JAHjatticel < JAHnydrationl When JAHlatticel is cole to |AHnydration There isn't enough information to determine.arrow_forward
- Please don't provide handwritten solution....arrow_forwardArrange the compounds from most soluble in water to least soluble in water. Most soluble in water Least soluble in water Answer Bank H2 H3C CNH2 H₂ H3C N C-CH3 H H₂ H₂ H3C C-N-C-CH3 CH3arrow_forwardWhat would the net equation be for sodium acetate trihydrate when we add acetic acid to produce a solution? (Buffer related)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY