
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
If this figure were extended one more “generation” down, how
many neutrons would be produced?
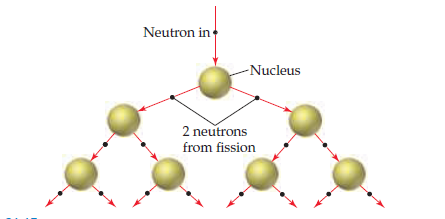
Transcribed Image Text:Neutron in
-Nucleus
2 neutrons
from fission
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the zone of stability? What are three decay processes that help an isotope get to the zone of stability?arrow_forward9. An unstable isotope that lies under the stability curve of the nucleus will emit.... a. electrons d. rays a (alfa) b. neutrons e. ray B (beta) d. positronarrow_forwardIn an atom, the mass number gives the number of ________ and _________ in the nucleus. Fill in the blanksarrow_forward
- BETA DECAY Write the symbol for the element produced when Strontium-90 undergoes beta decay... 240 94 Pu example ? ? ?arrow_forwardWhat Nuclear Fission and Nuclear Fussion? What the difference between these two?arrow_forwardGold has one stable isotope, whichis gold-197, with an atomic mass of 196.97amu. A 14k (14-karat) gold contains 58.3% of gold and 41.7% of other metals. How manyatoms of goldare present in a 5.00-g sample of 14k gold?arrow_forward
- Which of the selections completes the following nuclear reaction? y + Ca 40 20 3Ca+arrow_forward⁴solve with complete solution and explanationarrow_forwardWhat are the types of radiation emitted by the nuclei of radioactive elements? A) Helium nuclei B) B. B+ electrons and positrons, nuetrons y rays D y rays, B, ߆ electrons and positrons, nuetrons, and Helium nucleiarrow_forward
- Alpha particles are identical to Question 12 options: a) hydrogen atoms. b) electrons. c) protons. d) helium atoms. e) helium nuclei.arrow_forwardIsotopes are atoms with the same number of _______________ and _______________ but different numbers of ________________. (Place the words protons, electrons, and neutrons in the proper placesarrow_forwardNegatively charged particles of radiation emitted from the decay of radioactive substances are known as A) protons B) alpha particles C) beta particles D) neutrons E) gamma raysarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY