
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
7. Answer completely.
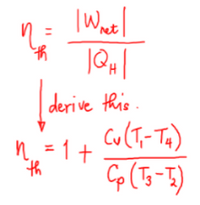
Transcribed Image Text:net
derive this.
Cu(T- Tx)
n=1+
th
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Voice Edit Determine the Miller-Bravais indices for plane shown below. ja2 d3 D'Focus 7:43 PM 62°F Saturday C Accessibility: Investigate 1/22/2022 (99+arrow_forwardSelect the most probable hazard associated with each operation. Group of answer choices compressor operation [ Choose ] flooding unburned hydrocarbons flame out working at heights noise agitation problem pressure surges high velocity steam cooling tower operation [ Choose ] flooding unburned hydrocarbons flame out working at heights noise agitation problem pressure surges high velocity steam reactor operation [ Choose ] flooding unburned hydrocarbons flame out working at heights noise agitation problem pressure surges high velocity steam flare operation [ Choose ] flooding unburned…arrow_forward8:34 PM Fri 5 May K 00 19CAPE10...0.. (b) Lo (i) (ii) X 19CAPE10200... X 1.82×10 18CAPE10200... -3 - 19CAPE102002s2 ✓ X 18CAPE10200.. X T @*** Answers to th.. O 0·013x = X Lecture 1 X 田 How long will it take until 99% of the reactant have reacted? 36% CAPE 1020 En. = 0'14x fac 2 600 s after initiation of a first order reaction 48.5% of the initial reactant concentration remains present. What is the rate constant for this reaction?arrow_forward
- (#), = V - T(), av Derive the relationshiparrow_forwardUsing the data below, construct a calibration curve. Include the error bars and the equation of the line and the linearity (R2) of the curve. Calcium concentration (ppm) (X-data) Absorbance (Y-data) Run 1 Absorbance (Y-data) Run 2 Absorbance (Y-data) Run 3 0.3 1109 1069 1155 1.2 1225 1168 1233 2.1 1472 1319 1389 4.2 1497 1523 1472 8.0 1833 1898 1849 16 2066 2012 2051arrow_forwardRemaining Time: 20 minutes, 12 seconds. * Question Completion Status: QUESTION 1 The dimensions of heat flux is ML2T -3 True False QUESTION 2 Dimensionless parameters are obtained using a method called dimensional analysis. O True ロFalse QUESTION 3 Click Save and Submit to save and submit. Click Save All Answers to save all answers. earcharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The