
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
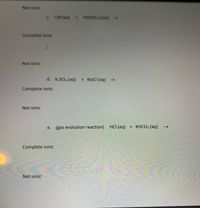
Transcribed Image Text:Net ionic
C. LIBr(aq) + Pb(NO,):(aq) -
Complete ionic
I
Net ionic
d. K,SO4 (aq)
+ NaCl (aq)
Complete ionic
Net ionic
e. (gas evolution reaction) HCI (aq) + KHCO3 (aq)
->
Complete ionic
Net ionic
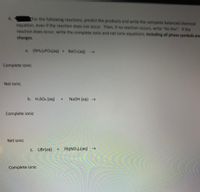
Transcribed Image Text:For the following reactions, predict the products and write the complete balanced chemical
equation, even if the reaction does not occur. Then, if no reaction occurs, write "No Rxn". If the
reaction does occur, write the complete ionic and net lonic equations, Including all phase symbols and
6.
charges.
a. (NHA) POa(aq) + BaCl(aq) >
Complete ionic
Net ionic
b. H2SO4 (aq)
+.
NaOH (aq)
Complete ionic
Net ionic
C. LiBr(aq)
Pb(NO3)2(aq) –→
Complete ionic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete, balance, and write the Q expressions: _Li(s) + _H2SO4(aq) <--> _______ (aq) + _H2(aq) Q = __________ _Na2SO4(aq) + _Hg2Cl2(aq) <---> __________(s) + ____(aq) Q = __________ _HCl(aq) + _NaHCO3(aq) <---> ____(aq) + ___(l) + CO2(aq) Q = __________arrow_forwardJj.8.arrow_forwardb. Balanced formula equation: HCNO (aq) + Complete ionic equation: + Net ionic equation: + + + KOH(aq) + +arrow_forward
- Q.2: For the following reaction : Na2SO4 (aq) + BaCl2 (aq) ? BaSO4 (s) + NaCI (aq) a- Write the balance molecular equation, Write the b- Write the complete ionic equation, C- net ionic equation.arrow_forwardcan you please do this and send an image of your work please and thanksarrow_forwardBarium chloride (BaCl2, aq.) reacts with sodium sulfate (Na2SO4, aq.) to produce barium sulfate (BaSO4, s) and sodium chloride (NaCl, aq.). Write: Molecular equation Complete ionic equation Net ionic equationarrow_forward
- F2(g) + SrCl2(aq) ---> SrF2(aq) + Cl2(g) Complete ionic: Net ionic:arrow_forwardWhich of these is a net ionic equation? a. Lit(aq) + Cl (aq) + Ag*(aq) +NO3 (aq) - -> AgCI(s) + Li* (aq) + NO3 (aq) b. LicI(aq) + AGNO3(aq) --> AgCl(s) + LİNO3(aq) c. Ag*(aq) + CI (aq) --> AgClI(s)arrow_forward[References] What volume of 0.0432 M calcium hydroxide is required to neutralize 34.20 mL of 0.0355 M nitric acid? Volume = mLarrow_forward
- HCIarrow_forwardExample: Write the molecular, complete ionic, and net ionic reactions between solutions of nitric acid and barium hydroxide. oxidized and what is being ed in the following redox 2 HCl(aq) CaCl(aq) ME: CIE: of redox reactions including combustion and single displacement is and decomposition reactions are also redox reactions. NIE: bondi nic charge! (But some lation numberarrow_forwardHelp with these pls!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY