
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
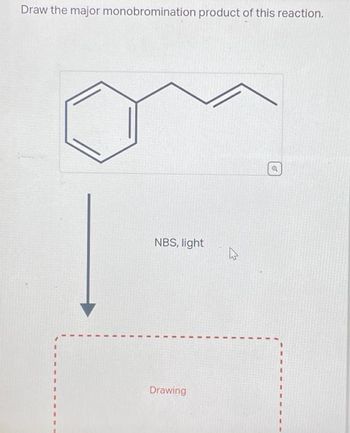
Transcribed Image Text:Draw the major monobromination product of this reaction.
NBS, light
Drawing
W
Q
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O ELECTRONIC STRUCTURE Interconverting the wavelength and frequency of... Stella X-rays have a wavelength small enough to image individual atoms, but are challenging to detect because of their typical frequency. Suppose an X-ray camera uses X-rays with a wavelength of 7.51 nm. Calculate the frequency of the X-rays. Round your answer to 3 significant digits. PHz da Explanation Check O 2020 McGraw-Hill Education. All Rights Reserved Torms of Use Privecy Accassibility 6:50 PM 11/8/2020 P Type here to search 国回arrow_forwardQUESTION 26 700-650 nm Red 649-580 nm Orange 579-575 nm Yellow 574-490 nm. Green 489-455 nm Blue 454-425 nm Indigo 424 - 400 nm Violet a) Which has the greater frequency, V, Red or yellow light? b) Which has the greater wavelength, Blue or yellow O a) yellow and b) blue O a) yellow and b) yellow O a) Red and b) yellow O None of abovearrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help Purple/Black Iridescent - KPM X wilmington postal code - Goo X 9 Dealers - Niche W A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZI6tTytly O ATOMS, IONS AND MOLECULES Counting protons and electrons in atoms and atomic ions Fill in the missing information: atom or ion? check all that apply symbol number of number of protons electrons O neutral atom O cation O anion 12 10 V neutral atom O cation O anion 48 Ge O neutral atom O cation M anion Explanation Check IIarrow_forward
- Consider the three electromagnetic waves shown in the image. Which wave has the longest wavelength? A Ов Which wave has the greatest frequency? B B Which wave has the greatest energy? Barrow_forwardplease he1parrow_forwardA baseball pitcher throws a fastball that moves at 95 miles per hour. Does thatmoving baseball generate matter waves? If so, can we observe them?arrow_forward
- any SI prefix in the ALEKS Data tab.) 1400 - 1200- 1000 800 energy (z) 600 400 200 Use this diagram to complete the table below. What is the energy of the electron in the ground state? What is the energy of the electron in the first excited state? If the electron makes the transition shown by the red arrow, from C to A, will a photon be absorbed or absorbed emitted emitted? Calculate the wavelength of the photon that would be absorbed or emitted. Round your answer to 3 significant digits. nm I Don't Know Submit © 2022 McGraw Hill LLC. All Righarrow_forwardor grading, click ön the Sub What is the shortest wavelength of light that can be emitted by a hydrogen atom that has an initial configuration of 7s1? Enter a wavelength in nm accurate to 3 significant figures. Number nm Submit Assignment Quit & Save Back Question Mearrow_forwardDraw a rainbow and arranged its color from the highest frequency to the lowest frequency.arrow_forward
- Please type all work Question 8 Explain the error in the following electron configuration for Sr2+, and provide the correct electron configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d2arrow_forwardGreen light has a frequency of about 6.00 × 1014 s1. What is the energy of a photon of green light? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HA ? ... Ephoton = Value Units Submit Part C Hospital X-ray generators emit X-rays with wavelength of about 15.0 nanometers (nm), where 1 nm 10-° m. What is the energy of a photon of the X-rays? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HA ? Ephoton Value Unitsarrow_forwarda) culate the first 3 electronic energy levels for He' and sketch an energy diagram for these levels. (this does not need to be to scale). b) Then label an electron relaxing from n = 3 to n = 2 on the energy diagram and calculate the energy of the photon that would be released. c) Based on the data collected during the atomic spectra lab, determine whether the photon emitted in the visible spectrum. Note, for He*, E = -2.18E-18 J * (4/n^2)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY