
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:|||
C
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-liG1Sp4C1qHE9vg-2-exSXq493r_tJ2gZs0ajljMpw3xX-Vf... A
O ELECTRONIC STRUCTURE AND CHEMICAL BONDING
Interconverting the wavelength and frequency of...
0
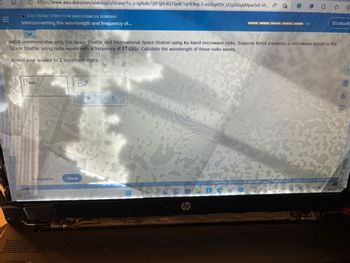
NASA communicates with the Space Shuttle and International Space Station using Ku-band microwave radio. Suppose NASA transmits a microwave signal to the
Space Shuttle using radio waves with a frequency of 17.GHz. Calculate the wavelength of these radio waves.
Round your answer to 2 significant digits.
#
Explanation
79°F
Mostly sunny
Check
0×10
X
S
14
A
O Search
hp
0/5
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
THIEP CHEROINSTER ***
C
55.98
Accessibility
FETH
Elizabeth
4) 10
3:53 PM
1/29/2023
olo
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvgXwPgmUhvITCeeBZbufuBYTi0Hz7m7D3ZSJ7UY-P6z3nNqF81EXBG_ O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Deducing valence electron configuration from trends in... The first five ionization energies (IE, through IE) of a Period 5 element have the following pattern: IE₁ IE2 E3 IE4 IE5 Make a reasonable guess about which element this is. Enter its chemical symbol below. Check X $arrow_forwardA www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFJQOAP_Ifnuy3jUYHEHEi1kH6Ggwtol-saQ_ImAyqsWT. O ATOMS, IONS AND MOLECULES Deducing the ions in a polyatomic ionic compound from its... Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation anion NaCl Na CI v (OH), NH,I CrF, Cu(No,), Explanation Check © 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use | Priva étv 6 IIarrow_forwardwww-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLlq7wcPWKzBYGfE9IMFjON2fAafz6ntlwYHfhDiQhHbeMUml7sVNJ_culZL2tO. O ATOMS, IONS AND MOLECULES Predicting and naming ionic compounds formed by two elements Iffa Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided. empirical formula of ionic compound element #1 element #2 Forms ionic name of ionic compound compound? oxygen calcium yes O no oxygen fluorine O yes O no magnesium cesium O yes O no calcium chlorine O yes O no Explanation Check O 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use | Privacy | Acce étv MacBook Air O OOO O OOO O Oo Oarrow_forward
- IgNSikr/J8P3]H-IQIHQRDYV_6Ux63SypJX O CHEMICAL BONDING Using the AXE notation to describe a molecule with a central... What is the "AXE" description of the trichlorostannanide ( SnCl, ) anion? AXarrow_forwardA ALEKS - Daniela Garcia Gonzalez X + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkasNW8D8A9PVVfeR1Y7GY2KsT3SAA8EqmvAn... O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Daniela Identifying the electron added or removed to form an ion For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you. atom subshell from which electron subshell to which electron removed to form +1 cation added to form -1 anion olo H 1s 1s Ar Li Ar Sc Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility 11:49 PM Type here to search 12/5/2021 18)arrow_forwardA ALEKS - Daniela Garcia Gonzalez X b My Questions | bartleby www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkasNW8D8A9PVVfeR1Y7GY2KsT3SAA8EqmvAnK... Q E O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Daniela Identifying the electron added or removed to form an ion For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you. subshell from which electron subshell to which electron atom removed to form +1 cation added to form -1 anion ol. H 1s 1s Ar K Ве Cd Explanation Check © 2021 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center | Accessibility 11:02 PM Type here to search 后 ) 12/5/2021 ...arrow_forward
- A ALEKS - Daniela Garcia Gonzalez X + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkasNW8D8A9PVVfeR1Y7GY2KsT3SAA8EqmvAnK... Q O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Daniela Identifying the electron added or removed to form an ion For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you. atom subshell from which electron subshell to which electron removed to form +1 cation added to form -1 anion H 1s 1s Y Не Si ? 11:31 PM Type here to search ( ) 12/5/2021 ... 18)arrow_forwardA ALEKS- Andr x ← → C ||| 1 67°F Grades for An x Clear atoms or ions www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-lvgXwPgmUhvlTCeeBZbufu O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Understanding periodic trends in atomic size Re-order each list in the table below, if necessary, so that the atoms or ions in it are listed in order of decreasing size. 2+ Al, Al²*, Mg F, I, C1 S, S², Ar Explanation Refugee Chor X atoms or ions in order of decreasing size 0.0.0 0.0.0 0.0.0 Check MTA Rear Tin X ‒‒ ‒‒ Q Search: 4 X Tiller barreto X 2 00 Pickup Truck xb Home | bartle x + BYTi0Hz7m7D3ZSJ7UY-P6z3nNqF81EXBG_06NckTAr70SXR... ☆ h 5 LO L 4 New Tab X 0/5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardeducation.wiley.com/was/ui/v2/assessment-player/index.html?launchld=06c032ef-b96c-4463-b835-41e474f31335#/question/23 How should the sulfur-oxygen bond lengths compare for the species So, SO2, So,, and so,?? 8Incorrect. Select the compound with the longest bond length. O SO3 O SO2 O so,2- O so.2 Hint Incorrect. Select the compound(s) with the second longest bond length(s). O SO3 O So2 O so,- O so, Assistance Used Hint 2 Incorrect. Select the compound with the shortest bond length. O So3 O SO2 O so,? O so,2arrow_forward
- tR-XZxj_1SoekWMaP1As1fEhnC179H4SICzl1mdWKpKlvbF3amiKazP. OF ORGANIC MOLECULES Drawing a Lewis structure for a simple organic molecule from a. GE OOD D Draw a Lewis structure of the molecule that matches the description below. All non-H atoms should have full octets, and all formal charges should be zero. Unless you're told otherwise, assume there are no rings in the molecule. Description: The molecule is composed of 10, 4H's, and 2C's and it contains a C-C single bond. Click and drag to start drawing a structure. 2021 McGraw-Hill Education All Rights Reseved Terms of Uhe Pcy Check Explanationarrow_forwardS Write electrc X uzdLQYhASX X G Predict the t X Periodic Tab X lb Answered: [ X OmySigTau M (r ow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take [References] a. Predict the molecular structure and bond angles for ICI5. Approximate bond angles are sufficient. Molecular Structure Bond Angles b. Predict the molecular structure and bond angles for SiF4. Approximate bond angles are sufficient. Molecular Structure = Bond Angles = %3Darrow_forwardMy NSU | Northeastern State Uni X Content Bb 3743900 learn-us-east-1-prod-fleet01-xythos.content.blackboardcdn.com/blackboard.learn.xythos.prod/58249d599753b/3743900?X-Blackboard-Expiratio.. Guest VSEPR formula Molecule Lewis structure Geometric shape NH2CI NH4+ CO2 SF4 PF5 2:59 IZarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning