
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
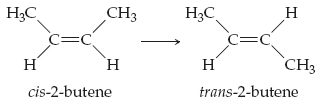
The molecule 2-butene, C4H8, can undergo a geometric change called cis-trans isomerization. (Figure 1) Such transformations can be induced by light and are the key to human vision. Rotation about the C=C bond, which breaks the π bond, is required for the isomerization.
The average bond energy (enthalpy) for a C=C double bond is 614 kJ/mol and that of a C−C single bond is 348 kJ/mol. Estimate the energy needed to break only the π bond of the double bond of 2-butene.
Express your answer numerically in joules per molecule.
Transcribed Image Text:НаС,
CH3
НаС.
Н
c=C
Н
c=C
CH3
trans-2-butene
Н
н
cis-2-butene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- To anyone who will answer this correctly, thank you.arrow_forwardThe reaction of Fe2O3(s) with Al(s) to form Al2O3(s) and Fe(s) is called the thermite reaction and is highly exothermic. What role does lattice energy play in the exothermicity of the reaction?arrow_forwardUse the molar bond enthalpy data in the table to estimate the value of AHin for the equation C₂H₂(g) + HBr(g) → → C₂H, Br(g) The bonding in the molecules is shown. +H-Br H- H H Br Bond O-H 0-0 C-O 0=0 C=0 C-C C-C C=C C-H C-F C-Cl C-Br C-N C=N kJ mol-1 464 142 351 502 730 347 615 811 414 439 331 276 293 615 Bond CEN N-H N-N N=N NEN F-F CI-CI Br-Br H-H H-F H-Cl H-Br H-S S-S kJ - mol-1 890 390 159 418 945 155 243 192 435 565 431 368 364 225arrow_forward
- The enthalpy change for the following reaction is -121 kJ. Using bond energies, estimate the C-H bond energy in CH,(g). CH,(g) + Cl½(g) →CH3CI(g) + HCl(g) kJ/molarrow_forwardUse bond energies (see table) to predict AH for the formation of HCI gas. H2(g) + Cl2(9) → 2 HCl(g) KJarrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Suppose there is an element X which occurs naturally as X2(g).X2(g) + 2O2(g) → X2O4(g)X2O4 has a structure ΔHof of O(g) is 249 kJ/molΔHof of X(g) is 474.5 kJ/molΔHof of X2O4(g) is 29 kJ/molThe X-X single bond energy is 160. kJ/molUse the above data to estimate the average bond energy in X2O4. Give your answer to the nearest 1 kJ/moarrow_forward2. The amide linkage is the connection between amino acids in the primary structure of proteins; a representation of the linkage is shown to the right: R2 (note: R1 and R2 represent the carbon centers of the respective amino acids) R1 H The expected bond angle around the Nitrogen is ~109°; the experimentally determined angle is 123°. What does this suggest about the C- N bond? (hint: consider possible resonance structures)arrow_forwardUse the average bond enthalpies from Table 8.3 in your textbook to estimate ΔHΔH for the formation of CO2(g) by the following reaction: CH4(g) + 2O2(g) --> CO2(g) +2H2O(g)arrow_forward
- a) The following reaction occurs in an open beaker: C2H5OH (g) ---> C2H4(g) + H2O (g) {Bond Energy (kJ/mol): C-H = 415 ; C-C = 345 ; C-O = 350 ; O-H = 464 ; C=C = 611}i) Calculate the ΔH0rxn for this reaction from the given bond energies. kJ ii) Is this reaction an open, closed or isolated system? iii) Would you feel hot or cold if you were near this reaction when it occurred? This is because (choose from I - V) I. the reaction is exothermic and absorbs energy from the surroundings. II. the reaction is exothemic and releases energy to the surroundings. III. the reaction is endothermic and absorbs thermal energy from the surroundings. IV. the reaction is endothermic and loses heat to the surroundings. V. the reaction produces steam which contains a lot of heat.arrow_forwardWhich of the following bonds are polar? (a) B¬F,(b) Cl¬Cl, (c) Se¬O, (d) H¬I. Which is the moreelectronegative atom in each polar bond?arrow_forwardBromomethane, CH;Br, is a very useful fumigant against a wide range of agricultural pests, from insects to the verticillium wilt fungus. Draw the Lewis structure of CH3Br.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY