
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
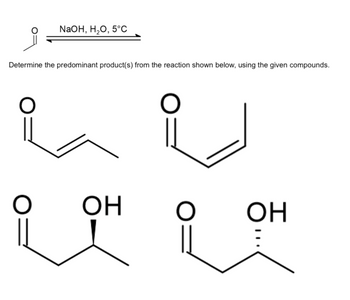
Transcribed Image Text:NaOH, 20, 5°C
Determine the predominant product(s) from the reaction shown below, using the given compounds.
O
ОН
O
он
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid, p-methoxybenzoic acid or p-(methylthio)benzoic acid? Explain.arrow_forwardGive detailed Solution with explanation neededarrow_forwardProvide the major organic product in the reaction shown below.arrow_forward
- Provide a detailed arrow pushing mechanism for the following reaction: ph Me ... NaOH, H₂O Me Me ph Mearrow_forwardWhen trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels, at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forwardProvide the major product of the reaction below. кино/ Heat COOH COOH сосн COCH COOH Darrow_forward
- Coumarin, a naturally occurring compound isolated from lavender, sweet clover, and tonka bean, is made in the laboratory from o hydroxybenzaldehyde by the reaction depicted below. Draw a stepwise mechanism for this reaction. Coumarin derivatives are useful synthetic anticoagulants.arrow_forwardTo preview the image click here Indicate which compound will have highest and lowest Bronsted acidity. 1. Choose the correct option for Highest Bronsted acidity [Select] 2. Choose the correct option for Lowest Bronsted acidity [Select] A B `ОН H3C :0: :0: تصور Darrow_forwardProvide the major organic product of the following reaction. Ph NaOH, Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning