
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
a 0.1 M solution of which of the following has a pH of 7.0?
see image
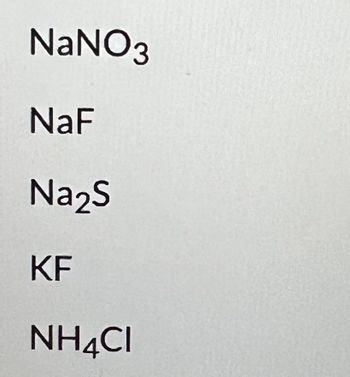
Transcribed Image Text:NaNO3
NaF
Na2S
KF
NH4CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the concentration of all species (H3PO4, H2PO4-) and the pH of a 0.200 M solution of H3PO4. a) What is the concentration (in Molarity) of H3PO4? b) What is the concentration (in Molarity) of H2PO4- ? c) What is the pH of H2PO4-?arrow_forwardGive correct explanation written answer onlyarrow_forwardPlease help I do not understandarrow_forward
- What mass of HI should be present in 0.250 L of solution to obtain a solution with pH value of 2.85. (A) 1.8 g (B) 0.035 g (C) 0.045 g (D) 0.055 garrow_forwardIf 5.0 mL of 0.40 M NaOH is added to 15.0 mL of 0.40 M HCl, what will be the pH of the resulting solution? • Round your answer to the nearest hundredth. Provide your answer below: pH =arrow_forwardCalculate the pH of a solution that is 0.66 M HNO, and 1.10 M KNO,. 3.372arrow_forward
- Please put in scientific not. if needed! Thank you:)arrow_forwardThe hand written answer is not allowedarrow_forwardWhat is the pH of an CuCl2 solution? (Hint: Split the compound apart into separate ions, determine if either is acidic, basic or neutral.) a) Not enough information given Ob) Acidic Oc) Basic O d) Neutralarrow_forward
- What is the pH of a solution made by mixing 25.00mL of .1500M NaOH with 25.00mL of .2500M HClO2? I am needing to make sure answer has correct pH significant digitsarrow_forwardH2. The pOH of an aqueous solution of 0.342 M quinoline (a weak base with the formula C9H7N) is . Please give typed answerarrow_forwardA solution contains 0.133 M NH4Cl and 0.199 M ammonia. The pH of this solution is Submit Answer Retry Entire Group 2 more group attempts remainingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY