
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
A picture of the two disaccharide repeats of hyaluronic acid are shown below.
Name the two different glycosidic linkages. Are these the same linkages as those found in glycogen?
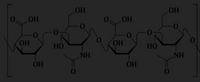
Transcribed Image Text:LOH
HO
HO
OH
OH
HO
HO
NH
NH
OH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Draw the glycosphingolipid with a head group (as Haworth projection) B-D-GalNac-(1->3)-ß-D-Glc-Sphingolipid. For the fatty acid, draw myristic acid.arrow_forwardWhat does this statement mean: "Upon folding of a protein many main chain amides replace their hydrogen bonds to water for those with other amide bonds" Is this true for alpha helix in proline racemase? and is there a pattern?arrow_forwardGiven the following pKa data for the individual amino acids, estimate the pI for the tripeptide Thr-Asp-Arg. N-terminal amino group = 9.10 Side chain of aa #1 = Side chain of aa # 2 = 3.86 Side chain of aa # 3 = 12.48 C-terminal carboxyl group = 2.01 PI =arrow_forward
- is it true that aplha and beta are made up of same amino acids but Beta chain is longer than alpha chain?what is other difference and similarity?is the amino aicd sequence exactly the same as well?are their structure the same ?arrow_forwardWith the aid of the simple generic diagram, identify and explain how the type of chemical bonding stabilizes a secondary structure present in 3GRS (glutathione reductase).arrow_forward(iii) Draw a structural diagram of a hydrogen bond between β-D-glucose and the side chain of any polar residue in a protein. (iv) Name the type of bond that can occur between the axial hydrogens of glucose and the indole ring of tryptophan. Draw a structural diagram of this bond. (v) Comment on the polar / apolar nature of the bond in part (iv) and explain why this type of interaction is prevalent in protein-sugar complexes. Answer (iii) to (v) please.arrow_forward
- Draw the structural formula of the oligopeptide if the amino acids are arginine, glutamine, glycine, methionine and glutamic acid considering the first amino acid is the N-terminusarrow_forwardDraw the tetrapeptide Met-Ile-Lys-Glu at a ph of 7?arrow_forwardPolylysine adopts a random structure in solution at physiological pH (i.e. 7.4). Given that the e-amino group of lysine has a pKa of 10.5, under what circumstances do you think polylysine will form an a-helix? Give an explanation for your decision. Given the pKa of the side-chain COOH group, under what circumstances would you anticipate polyglutamate to form an a-helix?arrow_forward
- Hemoglobin is considered to be a tetrameric complex with a 64 kDa (α β)2. When attempting to purify hemoglobin, we must first purify the α and β monomers (about 16 kDa each) to prepare the tetramer. This is formed from the dimer intermediate: 2 α + 2 β -> 2 αβ -> (α β)2. The graph given represents a size-exclusion chromatogram after the refolding of the hemoglobin tetramer Using the size-exclusion chromatogram given, 1. Draw an SDS-Page Gel with a reducing agent such as BME using the three peaks listed on the graph.arrow_forwardWhat would the following sequence of dodecapeptide M be? My best guess would be option Carrow_forwardConsider the peptide Trp-Arg-Glu-Cys-Gly-Tyr. For the drawings requested below, please show them in zig-zag style, from amino to carboxy terminus, with correct stereochemistry Draw the predominant form at pH = 2 Draw the predominant form at pH = 5 Draw the predominant form at pH = 7 Draw the predominant form at pH = 12arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON