
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
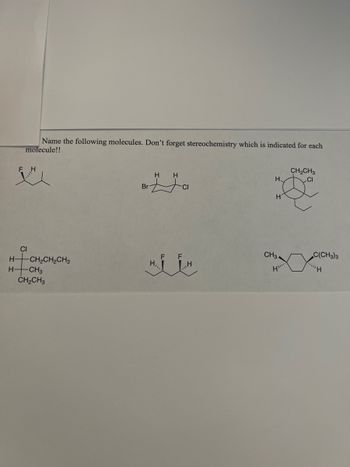
Transcribed Image Text:Name the following molecules. Don't forget stereochemistry which is indicated for each
molecule!!
F
H
H H
CH₂CH3
H.
CI
H
CH3.
H
CI
H-
H–|CH,CH,CH3
-CH3
CH₂CH3
Br
F
HIIH
H,,
н
C(CH3)3
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4 CI H3CH₂C Consider the following structure. Convert the Newman projection to a bond-line formula with wedges and dashes showing the stereochemistry. CH3 H3 CH ₂ C CI is cs protected and ma H F Polyclooctane R Please check if the diagram C is correct CH3 fap!!!! H K Harrow_forwardSubject :- Chemistryarrow_forwardCan you please please help me with these problems. Thank you so much!arrow_forward
- | 16. Which conformation of cyclohexane experiences the most transannular strain? a. chair b. planar c. boat d. twist boat e. All of these are stable. basdarrow_forwardname the following alkanes, and plz explain to me im a student:arrow_forwardIdentify the most stable chair conformation of the most stable isomer of 1,4-diethylcyclohexane. II II IV | || II IVarrow_forward
- 24. Trans-Decalin is more stable than its cis isomer, but cis-bicyclo[4.1.0] heptane is more stable than its trans isomer. Explain. H фф Н H trans-Decalin cis-Bicyclo[4.1.0]heptanearrow_forwardThe most stable conformation of 1,2-dibromoethane is: Select one: Br H. Br Br H. H. Br Br H. Br Br Br Br H.arrow_forwardEsc smooth 0 CH₂- Ph -CH-CH3 (3) (CH3)3C-C=C—CH(CH3)CH₂CH3 CH3 5c CH₂-C=C-C-OH T CH₂CH3 CH3 C C-CH₂CH3 53-methyloct-4-yne H₂C CH3 H C=C-CH₂CH₂ CH3 -CBr₂ C=C-CH3 CH,–C=C–CH3 Ph-C=C-H (CH₂)₂C-C=C—CH(CH₂) CH₂CH3 Chapter 9 Alkynes Quiz 2 1. Name each molecule. 2. Use the molecules in the reactions 1 through 9 highlighted in red to determine the product. 3. Draw the line/angle or skeletal structure of eachmolecule. 4. Use the alkyne below to predict the products 1- 9.arrow_forward
- 6. Sort the following conformations of 1-chloropropane from most stable to least stable. H CI Hн Н Н Н CH₂ н H н CI 2) Н CH, H 3) CH1 CIarrow_forward7.arrow_forwardOrganic Chemistry Мaxwell presented by Macmillan Learning Complete the most stable chair conformation of trans-1-tert-butyl-3-methylcyclohexane by filling in the missing atoms. Use the numbering provided on the ring. H Answer Bank 1 H. methyl tert-butyl H H H- 4 3 H H. CO 2.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY