
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
6
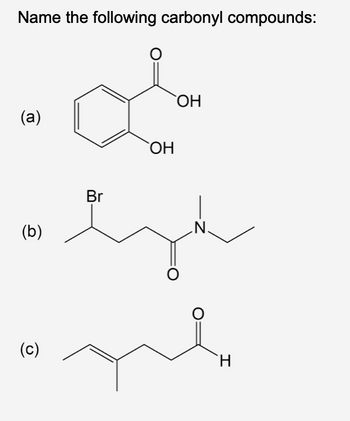
Transcribed Image Text:Name the following carbonyl compounds:
(a)
(b)
(c)
Br
OH
OH
O
N.
O
me
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide IUPAC names for (a) and (b). Provide the common name for (c) .arrow_forwardName these aldehydes:arrow_forwardWrite the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forward
- 6) The IUPAC name of this compound is: (CH3)2CHCOOH A) isopropylcarboxylic acid B) isopropanoic acid C) D) 2-methylpropanoic acid 2-methylbutanoic acidarrow_forward[10] 10] Q1. Do the following conversions as directed. Write the complete reaction equation. (a) Benzaldehyde to Benzoic Acid. (b) Propanal to 1-propanal (c) Cyclohexanone to Cyclohexanol (d) Acetaldehyde to Ethyl alcohol (e) Acetone to Iso-propyl alcoholarrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward
- Draw the structural formula for the major product of the following reactions.arrow_forwardName the following carboxylic acid derivatives, giving both a common name and anIUPAC name where possible. (c) PhCH(CH3)COOCH3arrow_forwardGive IUPAC names for the following compounds.(a) CH3OCH(CH3)CH2OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning