
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:A Classes
A Gas Laws HW1
Error
i classroom.google.com/u/0/c/MTQ2OTAONTE4MZE0/a/MZI00TIZMİMZODE1/details
SDPBC Bookmarks
E Maya Paniagua - CI.
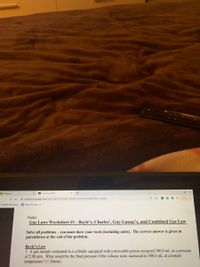
Name:
Gas Laws Worksheet #1 - Boyle’s, Charles’, Gay-Lussac's, and Combined Gas Law
Solve all problems – you must show your work (including units). The correct answer is given in
parentheses at the end of the problem.
Boyle's Law
1. A gas sample contained in a cylinder equipped with a moveable piston occupied 300.0 mL at a pressure
of 2.00 atm. What would be the final pressure if the volume were increased to 500.0 mL at constant
temperature? (1.20atm)
Transcribed Image Text:2. A balloon that contains 1.50 L of air at 1.00 atm is taken underwater to a depth at which the pressure is
3.00 atm. Calculate the new volume of the balloon. Assume that the temperature remains constant.
(0.500L)
Sign out
9 8 12:00
DELL
%23
2$
backs
e
y
u
d.
g
C
n
m
alt
ctri
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An ideal gas is allowed to expand from 2.00 L to 7.00 L at constant temperature. By what factor does the volume increase? factor: The pressure will increase by that same factor. decrease by that same factor. If the initial pressure was 111 atm, what is the final pressure? atm Pinal =arrow_forwardBoron trifluoride gas is collected at 1.0 °C in an evacuated flask with a measured volume of 15.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.420 atm . Calculate the mass and number of moles of boron trifluoride gas that were collected. Be sure your answer has the correct number of significant digits. mass: mole: molarrow_forwardMethane gas*arrow_forward
- 2. Pressure Conversions a. Convert 0.650 atm to mm Hg. b. Convert 710.0 torr to atm.3. Temperature Conversions a. Convert 30.0 Celsius to Kelvin b. Convert 25 Celsius to Kelvin4. Name 4 factors that affect the properties of gasesSolve the following using the gas equations. Temperature must always be converted to kelvin before using in any equation. In addition to Kelvin for the Ideal Gas Law, pressure must be in atmospheres, volume in Liters, and n in moles. The gas constant with units is R=0.08206 (L atm)/(n K). Notice that the units of R dictate which units must be used for each parameter. Mind the significant figures in your calculations.5. Boyle’s Law - Temperature constant a. A sample of gas has a volume of 400.0 mL when measured at 25oC and 760.0 torr. What volume will it occupy at 25oC and 195.0 torr? b. What final pressure must be applied to a sample of gas having a volume of 200.0 mL at 20.0 oC and 750.0 torr pressure to permit the…arrow_forward7. The partial pressure of CH4(g) is 0.175 atm and that of O2(g) is 0.250 atm in a mixture of the two gases. a. What is the mole fraction of each gas in the mixture? b. If the mixture occupies a volume of 10.5 L at 65°C, calculate the total number of moles of gas in the mixture. C. Calculate the total mass of the mixture (hint, find the mass of each gas in the mixture). с. d. Calculate the relative rates of effusion for molecules of CH4 to that of a molecules of O2.arrow_forward1. At 27°C and 720 Torr, a certain gas sample has a volume of 765 mL. a. What would be its new volume if the temperature and pressure were changed to 57°C and 792 Torr? Hint: Use Combined Gas Law. b. How many moles are present in that gas sample? Hint: Use Universal Gas Law.arrow_forward
- A sample of hydrogen gas that occupies a volume of 31.9 L at a temperature of 0 °C and a pressure of 1 atm contains ___ moles of gas.arrow_forwardWhich of the following should be done to reduce the pressure of 1 mole of an ideal gas in a 22.4 L container to zero torr? O Double the temperature of the gas. O Cool the gas to absolute zero. Halve the volume of the container. O Cool the gas to zero Celsius. Double the amount of gas.arrow_forwardA sample containing 4.70 gg of O2O2 gas has an initial volume of 11.0 LL . What is the final volume, in liters, when each of the following changes occurs in the quantity of the gas at constant pressure and temperature? A. A sample of 0.650 mole of O2O2 is added to the 4.70 gg of O2O2 in the container. B. A sample of 1.65 gg of O2O2 is removed from the 4.70 gg of O2O2 in the container. C.A sample of 4.80 gg of O2O2 is added to the 4.70 gg of O2O2 gas in the container.arrow_forward
- 13. A barometer is used to measure the total pressure of a mixture of gases A, B, and C. The total pressure is measured to be 750 torr. Assume the partial pressure of gas A is 203 torr and the partial pressure of gas B is 167 torr. What is the pressure of gas C? Do not include units with your answer.arrow_forwardA sample of nitrogen gas that occupies a volume of 45.5 L at a temperature of 0 °C and a pressure of 1 atm contains ________ moles of gas.arrow_forwardDoubling the initial pressure, from 1.0 atm to 2.0 atm, while keeping the temperature constant causes the volume of a gas to go from 1000 mL to a final volume ofarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY