
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
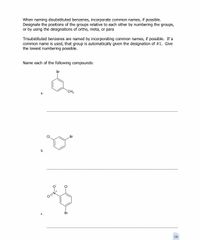
Transcribed Image Text:When naming disubstituted benzenes, incorporate common names, if possible.
Designate the positions of the groups relative to each other by numbering the groups,
or by using the designations of ortho, meta, or para
Trisubstituted benzenes are named by incorporating common names, if possible. If a
common name is used, that group is automatically given the designation of #1. Give
the lowest numbering possible.
Name each of the following compounds:
Br
CH3
а.
Br
b.
CI
O=N
C.
Br
16
Expert Solution
arrow_forward
Step 1
- Common names can be used in naming of compounds in chemistry.
- Example- Isobutane is the common name for 2-methylpropane.
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show Lewis Dot structures for the following compounds. a andb are ionic; c and d are molecular. For molecular compounds, the central atoms are shown in boldface. 4. a. Al2S3 b. CaBr2 С. OCS d. PH3arrow_forward3. Which of the following statements is true about the molecule shown below? 0- 0- O A. The bonds are polar and the molecule is polar. O B. The bonds are polar and the molecule is nonpolar. OC. The bonds are nonpolar and the molecule is polar. O D. The bonds are nonpolar and the molecule is nonpolar. 4. The image below shows a mixture of polar and nonpolar molecules. What type of intermolarrow_forward1. a. b. Br C. F F X F F d. Name the following molecules: e. -Farrow_forward
- 113. Name each of the following compounds: f. S,N4 g. SF4 a. Cul b. Cul2 coubone с. Col h. NaOCI d. Na,CO3 i. BaCrO4 j. NH,NO; e. NaHCO3arrow_forwardPls helo ASAP. Pls help in all asked questions.arrow_forwardWrite formulas for the following molecules: a. sodium bicarbonate b. chromium (III) nitrate c. lithium phosphate d. calcium sulfate e. iron (II) iodide f. potassium oxide g. phosphorus pentachloridearrow_forward
- Name the following compounds containing polyatomic anions g.(NH4)2SO3 h.(NH4)2S2O3 i.CsC2H3O2 j.CuCrO4 k.BaCO3 l.KMnO4 m.ZnCr2O7 n.Pb(BrO3)2 o.Hg2(BrO2)2 p.Na3PO4 q.KNO2 r.Ag2SeO4 s.Na2C2O4 t.Au(OCN)3 u.AuSCN v.Ni3(AsO4)2arrow_forward19. Identify the polyatomic ion in each of these ionic compounds. Write out the name and formula of the ions including their charges. CACO, Mg(OH), NH,CI 3. lon Names lon Charges Iarrow_forwardHelparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY