
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
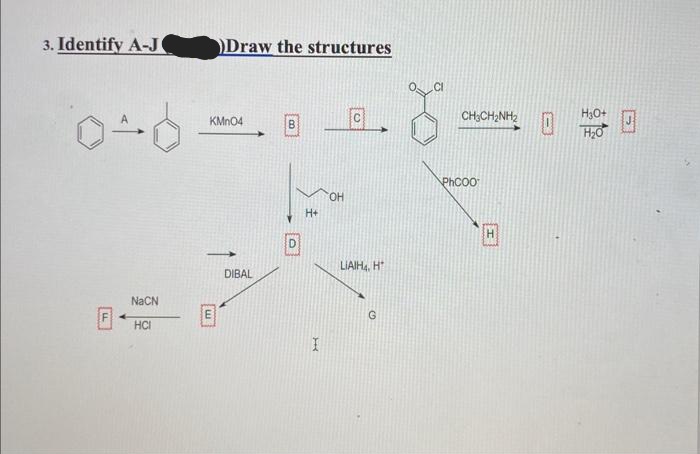
Transcribed Image Text:3. Identify A-J
040
NaCN
HCI
Draw the structures
KMnO4
m
DIBAL
D
H+
I
OH
с
LIAIH₁, H
G
CHỊCHINH 2
hcoo
H
H3O+
H₂0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Kapay X TEITE сартар го S Select the major product(s) for the following reaction. ОН only 1) BH3 THF 2) H2O2, NaOH H HO H + I н онarrow_forwardEndow Help eq req Ereg Important values if needed... | b... q eq MAY 11 Submit Answer prod01-cnow-owl.cengagenow.com S 5 According to the following reaction, how many moles of bromine monochloride will be formed upon the complete reaction of 0.915 moles bromine with excess chlorine gas? Br2(g) + Cl₂ (g) → 2BrCI(g) mol bromine monochloride Retry Entire Group ty A 6 [Review Topics] [References] Use the References to access important values if needed for this question. Module 2 | OWLv2 MacBook Pro & 7 8 more group attempts remaining GA * CO 8 A :)) O 9 COWLV2 | Online teaching and learning resource from C.... Previous 00 Email Instructor Thu May 11 Next Save and Exit 0 HARB 0 + C ?arrow_forwardCa* OH OH Ca* Ca* Ca* OH OH OH Ca* OH OH Ca OH OH Ca** OH Ca* 2+ Ca(OH) OH Саон A C Which beaker best depicts calcium hydroxide in a beaker of water? ов O A O D O Earrow_forward
- Please help me. Thank you!arrow_forwardbarkamishen seslb to doing Bittetsbau nA sideT oibore peivab ot etsimodo it as lendas suchombre or to agninnigod adr oldmseen polls anoitoleaimora ni baya Solovi immediabudinng enoitonen Isolmarlo lo coqyt Istoresbout wot ybuta lliw s Croborecho ofnino2s1 ayow ribi of 5) Analysis of 1.618 g of a hydrate salt gave the following information. Iliw Pb: 0.884 g, C: 0.205 g, H: 0.026 g, O: 0.273 g, and water: 0.230 g.o Inters What is the formula of the hydrate. sob a ogrobimu snocheoid bas in botser asce ed with asmolo s nad oson inmoasigaib to savi Inomalo srit 'il bogoo oinai is gaininos nouutos of babbe smogod bis noi si di autosis syneriozs Irw ticodulos ni noi ni nobnice to bilo 25ml produced flash and poppe carolionoriconic lieth noitonen inomossigen sigme yd elas Jabot ovi HA obrtw scale 6 10 enolonias pozemol adi ni ailuas, noitufos at devan gods got abou odregos bazum o obnoldo miuiboa bar otain rovlie to anoituloa asrl W SiT moironen insmoelor siduob a lo loss of an emot…arrow_forwardConsider the following chemical reaction: HCO3 + HCI ==== O HCO3 O HCI H₂CO3 O cr H₂CO3 + Cl. Which one of reactants is an acid?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY