
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
2 NA+Cl2->NaCl is the balanced reaction for the formation of table salt. Given 20 grams of Na and 10 grams of Cl2, which reactant is in excess? How much NaCl should be produced from these amounts of the two reactants?
If you could do it like how I’m being taught in class in this separate problem it would be much appreciated and would help me understand better
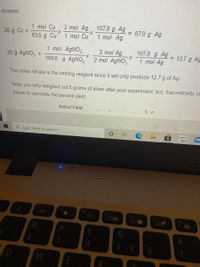
Transcribed Image Text:Answer:
107.8 g Ag
1 mol Ag
1 mol Cu
2 mol Ag
67.9 g Ag
%3D
20 g Cu x
1 mol Cu
63.5 g Cu
107.8 g Ag
X-
1 mol Ag
1 mol AGNO3
2 mol Ag
X-
2 mol AGNO3
= 12.7 g Ag
20 g AgNO3 x
169.8 g AGNO3
The silver nitrate is the limiting reagent since it will only produce 12.7 g of Ag.
Now, you only weighed out 5 grams of silver after your experiment, but, theoretically, yo
below to calculate the percent yield.
ActualYield
5 a
P Type here to search
amazon
Cocala
F6
F7
F8
OD
A
2
3
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the combustion of methane reaction, how many moles of O2 are needed to react with 7.80 moles of methane (CH4)? Assume you have an unlimited supply of O2. I need help with the amount on reactant side, element, amount on product sidearrow_forwardAn excess of sodium carbonate, Na2CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 12.51 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.arrow_forwardPart a) When FeS3 • 2H2O is heated, it gives off water, SO2 and Fe2O3. Is this a chemical or physical change? When CuSO4 • 5H2O is heated, it gives off water and CuSO4. Is this a chemical or physical change? Which of these changes can be easily reversed? Part b) Is the molar ratio of the products is different from the molar ratio in the hydrate formula, has a chemical or physical change taken place?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY