
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Add missing reagent(s) while also showing stereochemistry of the product of the second reaction.
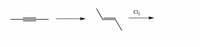
Transcribed Image Text:Cl,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What would the stereochemistry of the product of the second reaction be?
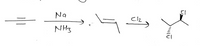
Transcribed Image Text:Na
NH3
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What would the stereochemistry of the product of the second reaction be?
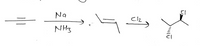
Transcribed Image Text:Na
NH3
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (c) NC. CN NC CN Aarrow_forwardh Oxidation: Cr2+ → Cr³+ + e Reduction: Cu²+ + 2 e¯ → Cu Net: 2 Cr²+ + Cu²+ What is the standard potential, Eºnet ? Standard Reduction Potentials Eº net Reduction Half-Reaction E° (V) Co³+ + e- → Co²+ 1.82 Au³ + + 3e- → Au(s) 1.50 Pd²+ + 2 e- → Pd 0.987 Ag++ e- → Ag(s) 0.80 Fe³+ + e- → Fe²+ 0.771 → Cu(s) 0.52 → Cu(s) 0.337 → Cut 0.153 → H₂(g) 0.00 → Sn(s) -0.14 Cu++ e- Cu²++2 e- Cu²++ e- 2H+ + 2 e- Sn²+ + 2 e- Co²+ +2 e- Cr³++ e- Fe²+ +2 e- Cr³+ +3 e- Zn²++ 2 e- A1³+ + 3 e- → Cu + 2 Cr³+ = V →Co(s) -0.28 → Cr²+ -0.41 → Fe(s) -0.44 → Cr(s) -0.74 → Zn(s) -0.763 →Al(s) -1.66arrow_forwardWrite a balanced redox reaction for the following electrochemical cell: Pt | Cu* (aq); Cu²+ (aq) || Fe3+ (aq) | Fe What is the correct balanced reaction for the cell?arrow_forward
- NaH MeO i Br [C] THE D E C8H1002 Aarrow_forwardCI 0 OCH 3arrow_forward2.95 Engineers who design bicycle frames are familiar with the densities of aluminium (2.699 g/cm3), steel (7.87 g/cm3), and titanium (4.507 g/cm3). How does this information compare with Figure 2.12, and what would it suggest for changes in this figure if more shades were used for the density colour-coding? (Iron is the principal component of steel)arrow_forward
- CH,2, Zn(Cu) Et,0 CH,l,, Zn(Cu) Et,0arrow_forwardQ = ([Li+]6 [Cl-]6)/([SmCl3]2) = (0.01006 * 0.01006)/(2.002) = 2.5 x 10-18 this calculation is wrong its 2.5 X 10-25arrow_forwardPlease draw an arrow pushing mechanism for the following transformation. Include all charges, lone pairs, and arrows. Short6.png ! (25kB)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning