
Intro Spectroscopy
5th Edition
ISBN: 9781305221796
Author: PAVIA
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Consider a magnet moving relative to a solenoid. Determine the direction of the induced magnetic field for the difference cases indicated in the table.
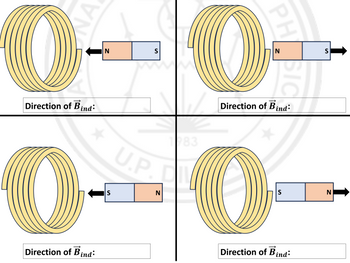
Transcribed Image Text:NA
N
S
PH
N
SIC
Direction of Bind
Direction of Bind:
S
983
U.P.DI
N
O
Direction of Bind
Direction of Bind
S
N
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- gnment Score: 68.3% x Give Up? Resources O Hint Check Ar stion 19 of 23 > Attempt- Classify the components as most accurately describing cofactors, coenzymes, or neither. Cofactors Coenzymes Neither Answer Bank Zn+ metal ion CoQ ascorbic acid FAD protease cer 6. garrow_forwardA.Use the bond energies to determine whether the following reaction is exothermic or endothermic and what is the amount of energy released or absorbed? H-O – O-H + H-O – O-H ---> O=O + H – O –H + H –O – H, hydrogen peroxide reacts to form oxygen gas and water. B. What are theBonds to Break, Bonds to Form?arrow_forwardoservation/ inference, include final moleculearrow_forward
- 1E.11 Magnesium ions form complexes with both ATP and ADP. The standard enthalpy of reaction for the hydrolysis of ATP to ADP and inorganic phosphate (PO3, which is denoted Pi) has been measured at pH = 7 and 25 °C for two different concentrations of magnesium ions: [Mg2+(aq)]/(mmol dm³) 0 1.0 Ahydrolysis He/(kJ mol¯¹) -21.7 -25.1 The standard reaction enthalpy for the formation of the complex between Mg 2+ and ADP is +18.0 kJ mol¯1. Calculate the standard reaction enthalpy for the formation of the complex between Mg2+ and ATP. Why does the presence of magnesium ions affect the reaction enthalpy for the hydrolysis of ATP?arrow_forward74.6% Resources x Give Up? E Feedback Resume Assignment Score: O Attempt 1 Question 25 of 28 > Write the molecular equation and net ionic equation for the reaction of hydroiodic acid and potassium hydroxide. Include phases (states). Enter the formula for water as H,O. molecular equation: KI(aq) + H,O(l) Incorrect net ionic equation: - H,Od) Incorectarrow_forwardAnswer letter c and darrow_forward
- Binding in solids Calculate the force of attraction between a Ca2 and an O2 ions where their centers are separated by a distance of 1.25 nm.arrow_forward0 X hs https://gz.explorelearning.com/index.cfm?method=cReso GPolarity and Intermolecular Force X HOPS L Type here to search 3. Which of the following statements is true about the molecule shown below? Ō+ C 6- OA. The bonds are polar and the molecule is polar. OB. The bonds are polar and the molecule is nonpolar. OC. The bonds are nonpolar and the molecule is polar. D. The bonds are nonpolar and the molecule is nonpolar. Con esource Part X + 70 resourceID=1091&ClassID=643519 22arrow_forwardHow do I solve this problem. Please give a step by step solution. Please do not use Chegg.arrow_forward
- No calc. Please follow required steps to better understand.arrow_forwardNo calc. Please follow required steps to better understand.arrow_forward(e) Using the characteristic function, show that a(N) (N²). = k„T (f) Show that fluctuations in the number of adsorbed particles satisfy (N*), (N)? 1-f Nfarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning