
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
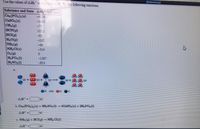
Transcribed Image Text:Use the values of AH to calulate AH for the follonng reactions.
Substance and State AH (LJ/mol)
Cag(PO,),(s)
C&SO (s)
CH,(G)
HCN(9)
HC(G)
H,0(g)
NH(9)
NH,CI(s)
0,(0)
HPO,(1)
H,80,(1)
4126
-433
-75
135.1
-92
-242
-46
-314
-1267
-814
(2) +
N OH 0O
AH"
b. Caa (PO), (s) + 31, SO.(1) + 3Ca$O.(s) + 2H,PO, (1)
c NH3 (g) + HCIG) NH, CI(s)
AH"=
kJ
888
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A typical recipe for a hot toddy (a drink often used as a cold remedy) is to mix hot water withwhiskey, honey, and lemon juice. A patient heats 16 oz. of 22C water on the stove until it hasabsorbed 33 kcal of energy. He then mixes 2.0 oz. of the hot water with 1.5 oz. of roomtemperature whiskey (d = 0.94 g/cm3, cs = 3.40 J/g C, T = 22 C). What is the temperature of thehot toddy (prior to adding the remaining ingredients)?arrow_forwardThe temperature of a sample of copper increased by 24.7 °C24.7 °C when 257 J257 J of heat was applied. What is the mass of the sample?arrow_forwardA 51.74 g sample of a substance is initially at 25.6 °C. After absorbing 2939 J of heat, the temperature of the substance is 184.5 °C. What is the specific heat (c) of the substance? c = J g• °Carrow_forward
- Use the following information to answer Questions 6-9: A sample of NH4NO3 with a mass of 5.0 g is dissolved in 40.1 g of deoinized water in a calorimeter. The initial temperature of the water is 21.2 oC. The final temperature of the solution is 15.4 oC. The calorimeter constant = 25.2 J/oC. If qH2O = -910 J and qcal = -130 J for 5.0 g of NH4NO3, calculate the heat of solution in J per gram of NH4NO3.arrow_forward45 kJ of energy are associated with dissolving a certain amount of solid sodium hydroxide (NaOH) pellets in water. If the temperature of the resulting solution rises, which option best describes this process? Н20 NaOH(s) > NaOH(aq) AH px = -45 kJ/mol %3D rnx Н20 NaOH(s) – → Na*(aq) + O2(g) + H2(g) AH, nx = -45 kJ/mol Н20 Na(s) + H20(1) –→ Na*(aq) + OH¯(aq) AH, -45 kJ/mol rnx Н20 NaOH(s) > NAOH(s) AHnx = +45 kJ/mol rnx Н20 NaOH(s) > Na*(aq) + OH(aq) AHmx = +45 kJ/mol %3D rnxarrow_forwardA 28.79 g sample of a substance is initially at 21.2 ∘C. After absorbing 1817 J of heat, the temperature of the substance is 161.0 ∘C. What is the specific heat (SH) of the substance? SH = _____ J/g⋅∘Carrow_forward
- 9.)arrow_forwardWhen a 8.47 g sample of sodium iodide, NaI, dissolves in 160.0 g of water in a calorimeter, the temperature rises from 20.5 to 22.5°C. The heat associated with this process is known as the heat of solution. What is qsolution for this reaction in kJ (not J) to 2 sig figs?arrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 0.38 kg sample of C,HgO, from 53.1 °C to 64.4 °C. The experiment shows that 6.73 × 10° J of heat are needed. What can the chemist report for the molar heat capacity of C,H,O,? Round your answer to 2 significant digits. | J- mol 1 - 1 ·Karrow_forward
- Energy cannot be created nor destroyed, but it can be transferred between a system and its surroundings. The change in internal energy, AU, is positive if the system absorbs energy, and it is negative if the system releases energy. (Figure 1) The total change in internal energy is the sum of the heat, q, and work, w: AU =q+w. Figure 1 of 1 Surroundings AU pystem u System >0 Surroundings AU system System u <0 AU = q + warrow_forwardCalculate the energy required to heat 593.0 g of cyclohexane from 11.1 °C to 35.6 °C. Assume the specific heat capacity of cyclohexane under these conditions -1 -1 is 1.85 J.g .K Round your answer to 3 significant digits. 0 0.0 X H 00 Sarrow_forwardWhen methanol, CH, OH, is burned in the presence of oxygen gas, O₂, a large amount of heat energy is released. For this reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH₂OH(g) + O₂(g) → CO₂(g) + 2 H₂O(1) AH = -764 kJ How much methanol, in grams, must be burned to produce 983 kJ of heat? mass: garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY