
What is the condensed and skeletal structure of the given lewis structure?
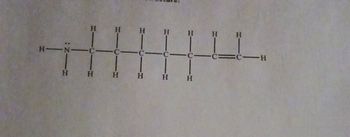
A condensed formula is a type of chemical formula that represents the atoms and bonds in a molecule using a minimal amount of symbols. In a condensed formula, the atoms are listed in the order in which they appear in the molecule, with the number of each type of atom indicated by a subscript.
In a skeletal structure, the molecule is represented as a simplified line drawing where carbon atoms are usually not explicitly shown. Hydrogen atoms bonded to carbon are also often omitted, and only the bonds between carbon atoms and other heteroatoms (like oxygen or nitrogen) are displayed.
Here we have to write the condensed and skeletal structure of the given molecule.
Step by stepSolved in 3 steps with 2 images

- What is the Lewis structure?arrow_forwardUse this condensed chemical structure to complete the table below.arrow_forwardTo answer the questions, interpret the following Lewis structure for SO42-. For the central sulfur atom: ... The number of lone pairs = The number of single bonds = The number of double bonds = The central sulfur atom: obeys octet rules or expanded octet rules or has incomplete octet rules? 2) To answer the questions, interpret the following Lewis diagram for CO2 .For the central carbon atom: ... The number of lone pairs = The number of single bonds = The number of double bonds = The central carbon atom: obeys octet rules or expanded octet rules or has incomplete octet rules? 3) To answer the questions, interpret the following Lewis structure for BCl3. For the central boron atom: ... The number of lone pairs = The number of single bonds = The number of double bonds = the central boron atom: obeys octet rules or expanded octet rules or has incomplete octet rules?arrow_forward
- How do you know when you need to add one or more multiple bonds to complete a Lewis structure?arrow_forwardUse Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forwarddraw the resonance structure of the molecule, and indicate the most stable form. Give the resonance hybrid at the endarrow_forward
- How many pairs ( set or two electrons together ) are in the Lewis for structure of carbon ?arrow_forwardConsider the most stable Lewis electron dot structure for CHNS containing no triple bonds. Draw this structurearrow_forwardThree Lewis structures for SCN are provided in Canvas. Circle the correct structure? Explain why it is correct and why the other two are incorrect.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





