
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Are my answers correct? Not too sure.
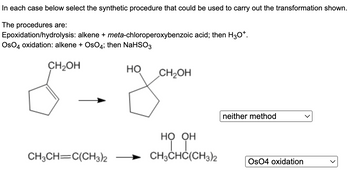
Transcribed Image Text:### Synthetic Procedures for Alkene Transformations
In each case below select the synthetic procedure that could be used to carry out the transformation shown.
**The procedures are:**
1. **Epoxidation/hydrolysis**: alkene + *meta*-chloroperoxybenzoic acid; then H₃O⁺.
2. **OsO₄ oxidation**: alkene + OsO₄; then NaHSO₃.
#### Transformations:
1. **First Transformation**
- **Starting Material**:
- Cyclopentene with a hydroxymethyl group (—CH₂OH) on the 1st position.
- **Product**:
- Cyclopentane with two hydroxyl groups (—OH), one where the double bond was and one on the 1st position, leaving the hydroxymethyl group (—CH₂OH) intact.
- **Correct Synthetic Procedure**:
- Neither method
- **Reasoning**:
- Neither epoxidation/hydrolysis nor OsO₄ oxidation will specifically produce the shown product. The transformation shown cannot be achieved with the given synthetic procedures.
2. **Second Transformation**
- **Starting Material**:
- 2,3-dimethyl-2-butene (CH₃CH═C(CH₃)₂)
- **Product**:
- 2,3-dimethyl-2,3-butanediol (CH₃CH(COH)(OH)C(CH₃)₂)
- **Correct Synthetic Procedure**:
- OsO₄ oxidation
- **Explanation**:
- OsO₄ oxidation is suitable for syn-dihydroxylation of the alkene, adding two hydroxyl groups to the same side of the double bond.
This selection process involves understanding the reactions by which these synthetic procedures transform alkenes into specific products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- i need the answer quicklyarrow_forwardSC Score: 0 of 1 point Incorrect Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. Steam reforming of methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 75.0 L tank with 14. mol of methane gas and 5.8 mol of water vapor, and when the mixture has come to equilibrium measures the amount of carbon monoxide gas to be 2.3 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. Answer Submitted: A K = 18 Submitted: Mar 2 10:23 PM Correct Answer: 0.0033 Assignments List ! F @ Review Assignment Time Spent: 39m 5s # с $ MacBook Pro G Search or type URL % & + © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use |…arrow_forwardMake a dilution series to get to 1:100. Start with making 1:10 dilution by adding 1mL of sample and 9mL of diluent, now continue the series to get to 1:100. The formula you may use is V1D1=V2D2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY