
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer the questions based on the following figure.
1.Draw the most likely ion fragment for the signals at m/z 77 and 185.
2.Draw the mechanism of formation for the ion fragment at m/z 130 and 228.
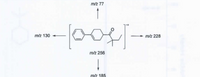
Transcribed Image Text:m/z 77
00-
m/z 130
m/z 228
m/z 256
m/z 185
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following IR frequencies would be expected for p-aminobenzoic acid? Select all that apply. O-3120 cm¹ (extremely broad; overlapping the C-H region) -1 ~3060 cm ¹ O~1675 cm-¹ ~3410 cm¹ (two signals) ~1475 cm ¹ and 1655 cm-¹arrow_forwardPlease list the appropriate peaks in the IR spectra results with a short narrative. Thank you.arrow_forwardWhy KBr used in IR spectroscopic sample preparation? Please shortly answer at your own words. Answer should be to the point (Specific 2-3 lines).arrow_forward
- Consider a molecule with the molecular formula C5H8O. Draw the structure of the molecule based on the data given.arrow_forwardplease draw the molecule and label it based on the data in the sheet and use the label in the data table.arrow_forwardValine is an essential amino acid. What peaks listed below would be expected in the IR spectrum of valine? Select the best answer from the choices below (not all peaks in the IR spectrum are listed). NH₂ OH Peaki- just over 3000 cm Peak: - just under 1000 cm Peak: Around so cm O Peaks and 2 O Peaks 1, 2 and 3 O Peaks 2 and 3 Peaks andarrow_forward
- Draw the most likely ion fragment for the signals at m/z 111, 139, and 156.arrow_forwardwhy are there two doublet signals in the aromatic region? use the structure of phenacetin POR O 4. The NMR of phenacetin is shown below: 181 ESPECTRUM 10 a) Why are there two doublet signals in the aromatic region? Use the structure of phenacetin in your answer. b) What hydrogen is represented by the signal at 7.9 ppm?arrow_forwardFrom the mass spectrum of Octane 1.Which peak represents the molecular ion? Label the peak with letter A at the top. 2. Which peak is the base peak? Label the peak with letter B at the top. 3. What is the name of the fragment responsible for the base peak? 4. Draw the structure of the fragment that produces the base peakarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY