
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw structures for the products in the following reactions.
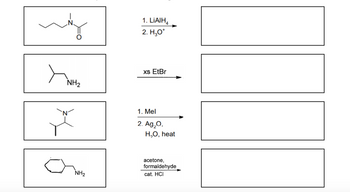
Transcribed Image Text:N.
may
NH₂
`N
NH₂
1. LIAIH
2. H₂O*
xs EtBr
1. Mel
2. Ag₂O,
H,O, heat
acetone,
formaldehyde
cat. HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A single alkyl bromide reactant theoretically yields either of the given products, depending on the reaction conditions. Draw the structure of the alkyl bromide compound. alkyl bromide (one compound only) CH, ОН СH O DMSOarrow_forwardWrite equations and answer questions for the following: Step 1: conversion of 1-bromobutane into a Grignard reagent. o Step 2: reaction of the Grignard reagent with the ketone to form an alkoxide salt. Identify the nucleophile (and its nucleophilic site) and electrophile (and its electrophilic site) in this reaction. O Step 3: protonation of the alkoxide salt using ammonium chloride as the proton source. Identify the acid and base in this reaction. o Write the net reaction.arrow_forwardFor the following reactions, draw the MAJOR product(s). 1) NaNH2 2) CH3I 3) 9-BBN 4) Н2О2, NaOнarrow_forward
- Draw the organic product of the following reaction.arrow_forward8) Show how the following conversions might be accomplished, including all reagents and intermediates. Br Y. OHarrow_forwardDraw a reasonable arrow - pushing mechanism that explains formation of the given products. HBr, A O Br Br, team teamarrow_forward
- Show how to synthesize the following product as the major product starting with 2,2-dimethylpropane as the starting material. You may use additional reagents and any number of steps. Be sure to list each step with all reactants/reagents/conditions required. (Do not use hydrogenation reactions). Write the process.arrow_forwardPlease help with thisarrow_forwardThe reaction of an alkyl chloride with potassium iodide is generally carried out in acetone to maximize the amount of alkyl iodide that is formed. Why does the solvent increase the yield of alkyl iodide? (Hint: Potassium iodide is soluble in acetone, but potassium chloride is not.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY