
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Multistep synthesis of Lidocaine Lab
1) How many grams of starting material do you need? It is given in moles below but how many grams?
2) How to calculate theoretical yield?
Part One Method
A. Preparation of alpha-Chloro-2,6-dimethylacetanilideCombine 0.033 mol of 2,6-dimethylaniline with 25 mL of glacial acetic acid (or ethyl acetateor THF) and 0.033 mol of alpha-chloroacetyl chloride, in that order, in an appropriately sized Erlenmeyer flask. With the aid of a hot water bath, warm the solution to 40-50°C, remove the flask from the bath, and add a solution of 5 g of sodium acetate trihydrate dissolved in 100 mL of water.
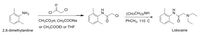
Transcribed Image Text:NH2
CI
(CH3CH2)2NH
CH3CO2H, CH3COONA
PҺCH3, 110 С
or CH3COOEt or THF
2,6-dimethylaniline
Lidocaine
IZ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You are in the quest to extract a drug from a known plant with medicinal properties. The structure of the compound is shown below: Which of the following solvents would be the best choice for extraction? Given solvents: Select one: A.) A B.) B C.) C D.) D E.) E F.) Farrow_forwardWhich of the following synthetic routes is used in industry to provide epichlorohydrin? Ca(OH)₂ C₂ H₂O a.1 b.2 c3 d.4 Cl₂ 500°C C₂ H₂O Ca(CH) Ch 500 ℃ Ca(OH)₂ Cl₂ 500 °C C₂ H₂O Cl₂ 500 °C Cl₂ H₂O Ca(OH)₂arrow_forwardI need hep writing these chemical equationsarrow_forward
- LAB 12: SYNTHESIS OF LIDOCAINE Experimental Procedure Step 2: Preparation of Lidocaine, 2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide – Step 1- Preparation of N-(2,6-Dimethylphenyl)chloroacetamide 1. To a pre-weighed 50-ml round bottomed flask, add the pressed-dry amide 1 and re-weigh the flask to get an exact amount of amide 1. 1. measure out 3.0 mL of 2,6- dimethylaniline into a 10-mL graduated cylinder and set aside in your hood. 2. sequentially add 7.5 mL diethylamine, 25 mL toluene and a few boiling stones. Attach a water-cooled reflux condenser to the round bottomed flask. 2. measure out 15-ml of glacial acetic acid and add it to a 125 mL Erlenmeyer flask. 3. Reflux the reaction mixture for 60 min. 3. Add the amine (2,6- dimethylaniline), via a Pasteur pipet, to the acetic acid in the Erlenmeyer flask. 4. Cool to room temperature, Transfer to a separatory funnel and wash the organic layer with 3x50 mL portions of 4. Transfer 2 mL of 2-chloroacetyl chloride to the Erlenmeyer…arrow_forwardSodium borohydride (NABH4) is a very selective reagent. Which functional groups can sodium borohydride reduce? Choose all that apply. O 1. Aldehyde II. Ester III. Carboxylic Acid O IV. Ketonearrow_forwardWhat is the ratio of benzaldehyde to thiamine hydrochloride used in this reaction? Why are we not using a full equivalent of this reagent?arrow_forward
- CH3CH2CH2CH2Cl + 1) KCN 2) LiAlH4 3) H2O yields ___________. cyclopentylnitrile pentan-1-amine butan-1-nitrile pentan-1-nitrile 1-chloro-butan-1-nitrile Thank you!arrow_forwardglowe OMe Selected Answer: Answers: 1. HO 2. EtMgBr 3. H3O+ 1. HOT 2. EtMgBr 3. NaOH EtMgBr, followed by aqueous workup 1. HO 2. EtMgBr 3. H3O+ ? 1. LIAIH4 2. EtMgBr 3. NaOH OH OH OH OH O OMearrow_forward#1arrow_forward
- 8. N-Phenethylmorphine was synthesised from morphine and found to have enhanced activity. Why does N-phenethylmorphine have higher activity than morphine? lina> HO HO HO но Morphine NPhenethymorphine a Increased binding to an extra binding region by van der Waals interactions. b. Increased binding to an extra binding region by hydrogen bonding c. Increased binding to an extra binding region by covalent bonding d. Increased binding to an extra binding region by ionic bondingarrow_forwardSynthesis of Dilantin by Multi-Step Synthesis Part 4 - Ureabenzil Condensation Table of Reagents Molecular weight (g/mol) Mass (g) Volume (mL) density (g/mL) Amount (mmol) Reagent benzil 210.23 1.0 4.75 Sodium hydroxide (30% wv) 39.997 1.2 loo urea 0.432 7,19 - Limiting Reagent: Identify the limiting reagent (warning, some reagents may be catalysts, and can be used in sub- stoichiometric amounts, and not all reactions have 1:1 stoichiometric coefficients) Yield of Product: Calculate the % yield of product you obtained. Watch significant figures and SHOW YOUR WORKING. Overall yield of product based on benzaldehyde: Calculate the % yield of the product you obtained based on the starting material for this synthesis, benzaldehyde. Watch significant figures and SHOW YOUR WORKING.arrow_forwardWhat compound results from the reaction below? NH₂ quaternary ammonium salt (three CH3I add to analine) primary amine amide nitrous acid CH3I (excess) ammoniaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY