
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
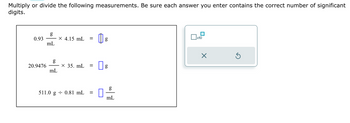
Transcribed Image Text:Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant
digits.
0.93
20.9476
g
mL
g
mL
X 4.15 mL
x 35. mL =
511.0 g 0.81 mL =
0
g
g
mL
x10
X
Ś
Expert Solution
arrow_forward
Step 1: Introduction to the given data
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 11 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forwardAdd or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 9.500 g 0.67 g = 9.60 g 1.2 g 12.70 g + 1.070 g = = ☐g g x10 X Undoarrow_forwardPlease don't provide handwriting solutionarrow_forward
- Using the table of equalities, calculate the number of cups in 1.0 gallons (gal). Remember to select an answer with the correct number of significant figures. Some Common Equalities Quantity Metric (SI) Length Volume Mass Time 1000 m 1 km Im= 1000 mm 1 cm 10 mm IL 1000 mL 1 dl = 100 mL 1 mL = 1 cm³ 1 kg = 1000 g 1 g = 1000 mg 1 h = 60 min 1 min = 60 s U.S. 1 ft = 12 in. 1 yd = 3 ft 1 mi 5280 ft 1 qt = 4 cups 1 qt=2pt 1 gal - 4 qt 1 lb = 16 oz 1 h = 60 min 1 min = 60 s Metric-U.S. 2.54 cm 1 in. (exact) I m = 39.4 in. 1 km 0.621 mi 946 mL- 1 qt 1L = 1.06 qt 1 kg = 2.20 lb 454 g 1 lb 12 cups 16 cups 64 cups 8.0 cups I DON'T KNOW YETarrow_forwardAdd or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 14.820 mL + 3.97 mL = 9.77 mL 6.9 mL = 2.70 mL + 6.800 mL = mL mL mL םיים × Śarrow_forwardWhen converting between metric units, use the prefixes to help you determine the magnitude of a value. The prefix kk indicates kilo, 1000. Therefore 1000 gg = 1 kgkg. A 25.0 kgkg iron weightlifting plate has a volume of 3180 cm3cm3 . What is the density of the iron plate in g/cm3g/cm3?arrow_forward
- Calculate the density of magnesium metal if 860 cm3 block has a mass of 1.51 x103g. Express the answer with the correct number of significant figures. 0.569 cm3/g 0.60 g/cm3 0.60 cm3/g 1.8 g/cm3 1.76 g/cm3arrow_forwardHow many significant figures should your result contain? What is the calculated density? Express your answer to the correct number of significant figures.arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 20.94 cm x 80 cm - 93. g ml. -* 19. mL - 946.2 mol 31.5 L - 0 cm 2 mol 0.9 Xarrow_forward
- Do not give handwriting solution.arrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 93. g/mL×38.mLarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 363.3 mol 0.63 L 542.6 mol 32.72 L 7.8084 cm X 2.125 cm 10 mol L mol L cm² x10 Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY