
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
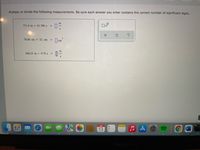
Transcribed Image Text:Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.
m
711.4 m + 61.780 s
%3D
78.08 cm x 32. cm
%3D
cm
846.25 m + 0.79 s
13
MacBod
E| n
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Add or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 1.507 mL + 0.8 mL mL x10 19.6 mL + 8.870 mL | mL 8.577 mL 4.77 mL mLarrow_forwardThe dimensions of a rectangular object were obtained as shown below. Use the data to determine the area of the rectangle. Make sure your final answer is reported to the correct number of significant figures: Width 3.56 cm 1sam Length 5.425 cmarrow_forwardCalculate the mass of gold (III) chloride (Au₂Cl) that contains a billion (1.0 × 10³) gold atoms. Be sure your answer has a unit symbol if necessary, and round it to 2 significant digits. 00 x10 X 3arrow_forward
- Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 2.09476 cm X 3.325 cm 444.8 m 0.35 s = 20.9476 cm X 43. cm cm m 2 S cm x10 Xarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 783.6m÷0.58sarrow_forwardAdd or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 19.770 g 0.9 g 6.50 g +1.500 g 6.50 g - 0.6 g = = 8 x10 X Undoarrow_forward
- Add or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 8.927 mL + 6.77 mL = 3.570 mL + 18.8 mL = 17.51 mL 14.9 mL = 0₁ mL 0 mL mL 2⁰0 Xarrow_forwardAdd or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 5.97 g 0.5 g g 9.800 g + 0.9 g g 4.87 g 0.570 g %3Darrow_forward42.8 grams of zinc shot is added to 12.5 ml of water in a graduated cylinder. The water rises to the 29.6 mark. From this information, calculate the density and specific gravity.arrow_forward
- Add or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 9.500 g 0.67 g = 9.60 g 1.2 g 12.70 g + 1.070 g = = ☐g g x10 X Undoarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits esc 274.4 m ÷ 61.739 s = 235.87 mol + 0.54 L = 7.808 8 mL Expmation x 2.825 mL = Check W 0 mol L 0 g > H E R Costa X 5 MacBook Pro I 2022 McGraw Hill LLC. All Rights Reserved. Te You *arrow_forwardUsing the table of equalities, calculate the number of cups in 1.0 gallons (gal). Remember to select an answer with the correct number of significant figures. Some Common Equalities Quantity Metric (SI) Length Volume Mass Time 1000 m 1 km Im= 1000 mm 1 cm 10 mm IL 1000 mL 1 dl = 100 mL 1 mL = 1 cm³ 1 kg = 1000 g 1 g = 1000 mg 1 h = 60 min 1 min = 60 s U.S. 1 ft = 12 in. 1 yd = 3 ft 1 mi 5280 ft 1 qt = 4 cups 1 qt=2pt 1 gal - 4 qt 1 lb = 16 oz 1 h = 60 min 1 min = 60 s Metric-U.S. 2.54 cm 1 in. (exact) I m = 39.4 in. 1 km 0.621 mi 946 mL- 1 qt 1L = 1.06 qt 1 kg = 2.20 lb 454 g 1 lb 12 cups 16 cups 64 cups 8.0 cups I DON'T KNOW YETarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY