
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
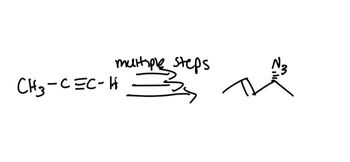
Transcribed Image Text:М
w
- H-2=0-Ено
scats adstimmu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- *54. A compound called di-t-butyl peroxide [abbreviation DTBP, formula (CH3);COOC(CH3)3] decomposes to give acetone [(CH3),CO] and ethane (C,H,): (CH3); COOC(CH3)3 (g) → 2 (CH;)2CO(g) + C2H6 (g)arrow_forwardCGE 32023#/ Dr. W. Scott Pers... Part C m = Submit Part D ΔΗ = How many grams of MgO are produced during an enthalpy change of -232 kJ ? Express your answer in grams to three significant figures. 0/ IVD| ΑΣΦ Submit Provide Feedback Krause etal_2007... Paraphrasing Paraphrasing Request Answer MacBook Air W aaac 2 0 0 ссссс VE ΑΣΦ Request Answer C 2. Ć Tool... Tool... ? How many kilojoules of heat are absorbed when 40.8 g of MgO(s) is decomposed into Mg(s) and O2 (g) at constant pressure? Lxpress your answer in kilojoules to three significant figures. 60 ? g X C C Ⓒ ✰ ✰ ☐ kJ Sentence Checker... | Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Contact Us | + Update Next >arrow_forwardKk.404.arrow_forward
- Step 3 of 8 Sum the equations for d[H] and d[Br] and solve for [Br]. (Use the following as necessary: [Br2], [H], [H2], [HBr], k1, k2, k3, k4, and k5.) dt dt [Br] = K5 2k1 [Br.] 2k [Br2] ks Step 4 of 8 Using the equation for I from Step 2, solve for [H]. (Use the following as necessary: [Br], [Br2], [H2], [HBr], k1, k2, k3, k4, and k5.) dt ky[ Br][H,] kg[Br] + k4 [HBr] [H] = k2[Br][H2] k3[Br2] + k4[HBr] Step 5 of 8 Substitute the equation for [Br] from Step 3 into the equation for [H] from Step 4 and simplify. (Use the following as necessary: [Br2], [H2], k1, k2, k3, k4, and k5.) k, k5 [Br.] [H] k3[Br2] + k4[HBr] Submitarrow_forwardΗ steps O= Η + diastarrow_forwardH -I H `H I H₂, Pd/Carrow_forward
- Br OH OH 14. H. (1) (II) Conversion of I to II : (a) takes place by Sy! (c) takes place both by Sy1 and S (b) takes place by Sy² (d) does not take placearrow_forwardtarrow_forwardX D2 Grades - CHE1011C41 Intro to CX signmentProblemID=195546712 100 11 Part A O exothermic reaction O endothermic reaction Classify reaction as exothermic or endothermic. Gas burning in a Bunsen burner: CH4(g)+20₂ (g)→CO₂ (g)+2H₂O(g) +802kJ Submit Part B Give AH for reaction above. Express your answer as an integer. Request Answer VE ΑΣΦ 1 Submit Part C Request Answer MasteringChemistry: Chapter 7 X + * lii ? kJ NO & Review Constants 43°F ^ @ (4¹) 2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY