
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How to find moles and molecules/atoms for C12H22O11and FE and H20

Transcribed Image Text:Molar Mass Lab
Introduction:
The mole represents a number. It is the number 6.022 x 1023. A mole of hydrogen atoms
contains 6.022 x 1023 hydrogen atoms. A mole of water contains 6.022 x 1023 molecules of
water. A mole of carbon dioxide contains 6.022 x 1023 molecules of carbon dioxide.
Objective:
To determine which of the samples on your table contains the most atoms.
Procedure:
Determine the mass of each element on your table. Be gentle when placing objects on the
balance. Record your data on the table
Station 1- Table Sugar
Measure a teaspoon of table sugar.
2. Calculate the molar mass of table sugar (a.k.a. sucrose - C12H22O11)
3. Now, weigh the table sugar in your scoop. Record the weight.
4. Calculate the moles of table sugar in the teaspoon.
5. Calculate the amount of molecules in the teaspoon of table sugar. (Hint: Use your answer
from #4 as your starting point!)
1.
Station 2- Nails/Screws
6. Find a nail or a screw.
7. Calculate the molar mass of Fe.
8. Now, weigh the nail/screw. Record the weight.
9. Calculate the moles of Fe in the nail/screw.
10. Calculate the amount of molecules in Fe. (Hint: Use your answer from #9 as your starting
point!)
Station 3- Water
11. Measure a % cup of water.
12. Calculate the molar mass of water.
13. Now, weigh the water. Record the weight.
14. Calculate the moles of water in a % cup.
15. Calculate the amount of molecules in the 4 cup of water. (Hint:
your starting point!)
your answer from #14 as
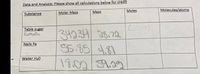
Transcribed Image Text:Data and Analysis: Please show all calculations below for credit
Moles
Molecules/atoms
Substance
Molar Mass
Mass
Table sugar
C.
34334 36.72
55.85 481
18:029.22
C12H22011
Nails Fe
Water H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- nment/takeCovalentActivity.do?locator=assignment-take t Visited [Review Topics] [References] Use the References to access important values if needed for this question. The formula for carbon dioxide is CO₂. a. How many molecules are in 7.68 grams of carbon dioxide? molecules b. What is the mass in grams of 1.01 x 1023 molecules of carbon dioxide? grams Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardCalculate the mass percent composition of carbon in C2H4O2 and CH4 to determine which compound has the most carbon in it by mass a) C2H4O2 b)CH4arrow_forwardWhat is a molecular formula and a empirical formula of a molecule C4H10O2arrow_forward
- How many H atoms are there in 40 molecules of C6H6?arrow_forwardExcedrin headache pain relief extra strength also contains 65 mg of caffeine, C8H10N4O2. How many hydrogen atoms does it contain?arrow_forwardhich of the compound(s) listed below is/are most likely a olecular compound? 1) CaBr2 II) CF4 III) MgO IV) PC|3 O II and IV I, II, and III I and III I only IV onlyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY