
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
***Molecule: 7-chloro-4-ethylhept-2-ene
Assignment 5: Separation Scheme Correction
- On a page titled Incorrect Separation Scheme print the incorrect separation scheme provided for your molecule on Blackboard. The top of the separation scheme shows what other compound is mixed with your molecule. Assume for the purposes of this assignment that both compounds are solid at room temperature. Also assume that both compounds are soluble in ether, except ionic compounds. The goal of the separation is the isolate each of the two compounds from the mixture.
- Below the incorrect separation scheme write a discussion of this incorrect scheme identifying all of the mistakes in the separation scheme. Keep in mind that there will be more than one mistake in the scheme.
- For each mistake, give a detailed, scientific explanation of why it is incorrect.
- On a page titled Correct Separation Scheme print/draw a correct separation scheme to isolate your molecule from the provided second molecule. Make sure to indicate the phase of each chemical in every step (s, l, g, aq) and write the separation technique used in each step (ie: vacuum filtration). The list below gives the reagents available in the lab. Use any of the reagents below to isolate the compounds. Remember that the simplest separation scheme is the best one. Try to avoid unnecessary steps.
|
· 10% aqueous NaOH |
· H2O |
|
· 5% aqueous NaHCO3 |
· H3PO4 |
|
· 6M HCl |
· anhydrous Na2SO4 |
|
· 5% HCl |
· anhydrous MgSO4 |
|
· Diethyl ether (ether) |
· Saturated NaCl |
|
· CH3OH |
· Sodium borohydride (NaBH4) |
|
· lithium aluminum hydride (LiAlH4) |
· NaCl solid |
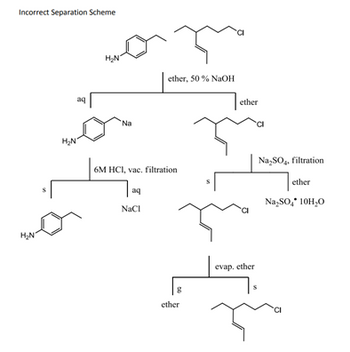
Transcribed Image Text:### Incorrect Separation Scheme
#### Description:
The diagram illustrates a chemical separation scheme that is identified as incorrect. It shows a series of reactions and separations involving multiple solvents and reagents. Below is a detailed breakdown of the diagram:
#### Steps and Reactions:
1. **Initial Mixture**:
- The starting mixture contains two compounds:
- A benzene ring with an amine group.
- A chloro-substituted alkane.
2. **Addition of Ether and 50% NaOH**:
- The compounds are mixed with ether and 50% sodium hydroxide (NaOH).
- This separates into two layers:
- An aqueous (aq) layer containing the sodium salt of the amine and the organic layer in ether containing the chloro-substituted alkane.
3. **Separation into Layers**:
- The aqueous layer contains the sodium salt of the amine.
- The ether layer contains the chloro-substituted alkane.
4. **Acidification**:
- The aqueous layer is acidified with 6M hydrochloric acid (HCl) and vacuum filtered. The benzene ring with the amine group precipitates.
- The organic layer is also separated.
5. **Washing and Drying**:
- The ether layer is washed with sodium sulfate (Na2SO4) to remove any water.
- After filtration, the sodium sulfate contains the water.
6. **Evaporation of Ether**:
- The ether is evaporated, leaving behind the chloro-substituted alkane as the final product in the solid (s) phase.
#### Diagram Analysis:
- **Incorrect Aspects**:
- The diagram does not show efficient separation procedures.
- The overlap and incorrect handling of phases might cause impurities in the final products.
- The process steps might not be sequentially correct, leading to possible experimental errors.
This separation scheme demonstrates complexities in ensuring compounds are correctly separated and highlights common pitfalls in chemical separation procedures.
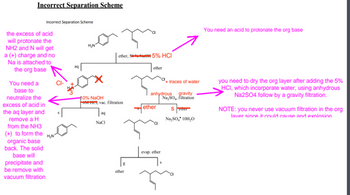
Transcribed Image Text:# Incorrect Separation Scheme
### Diagram Explanation
The diagram is labeled as an "Incorrect Separation Scheme," illustrating a chemical separation process. The scheme includes various chemical structures and actions associated with separating organic and inorganic substances.
### Labels and Annotations
The diagram contains several chemical separation steps, each annotated with corrections and explanations. The annotations include:
1. **Protonating the Organic Base**
- "You need an acid to protonate the org base."
- The process starts with an organic base in ether, and moves towards the addition of 5% HCl, which is incorrectly struck through and corrected next to it.
2. **Basification With NaOH**
- "You need a base to neutralize the excess of acid in the aq layer and remove a H from the NH3 (+) to form the organic base back. The solid base will precipitate and be removed with vacuum filtration."
- Indicating the use of 10% NaOH (incorrectly neglected initially) to remove a proton and revert NH3+ back to NH2, resulting in precipitation for filtration.
3. **Drying the Organic Layer**
- "You need to dry the org layer after adding the 5% HCl, which incorporate water, using anhydrous Na2SO4 followed by a gravity filtration."
- Highlights the need to remove traces of water using anhydrous Na2SO4, followed by gravity filtration.
4. **Safety Precaution**
- "NOTE: you never use vacuum filtration in the org layer since it could cause an explosion."
- Advises against using vacuum filtration in organic layers due to the risk of explosion.
5. **Arrows and Annotations**
- Various steps in the process are linked by arrows.
- Incorrect steps are struck through, and correct steps are annotated highlighting necessary changes.
### Summary of Steps
1. **Initial Addition of HCl**
- Begin with the organic base in ether.
- Add 5% HCl to protonate, though care must be taken to correct the improper labeling in the scheme.
2. **Neutralization with NaOH**
- Separate the aqueous layer containing the acid-base conjugate.
- Add 10% NaOH to precipitate the solid organic base having removed excess proton.
3. **Drying the Organic Layer**
- Use anhydrous Na2SO4 in the organic layer to dry it by removing traces of water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help in answering the organic chemistry questions given below. Explanations are welcome.arrow_forwardThe second question was incorrect. "The IUPAC nomenclature of benzene compounds primarily will use benzene as the root name. However, common names for substituted benzene compounds exist that use the base names from its derived compounds. The principal functional group will serve as the common root name." Would you mind trying again please? Thank youarrow_forwardEach of the following reactions has been reported in the chemical literature and gives a pre- dominance of a single product in synthetically acceptable yield. Write the structure of the prod- uct. Only monosubstitution is involved in each case, unless otherwise indicated. ***Draw the line structures in their entirety.*** C(CH3)3 CH(CH3)2 CH(CH3)2 NO₂ HNO, H₂SO4 HNO, acetic acidarrow_forward
- Indicate as "True" each of the following factors that are reasons why 2-bromopropane was added in excess. 2-Bromopropane is volatile, and losses due to evaporation are likely. O True O Falsearrow_forwardFor the compound below, draw the expanded, condensed, AND line structural formula. Also write the properties of the compound shown below. For the properties, make sure to include the reactivity, molecular geometry, bond angle, polarity, solubility and IMF. Lastly, briefly describe how you determined the structural formulas and properties. Thank you. 2 - ethyl-4- methylpentan-2 - olarrow_forwardOrganic chemistry student Karen is tasked with synthesizing (S)-3-methylbutan-2-ol. Using her knowledge of alkene reactions, she runs a hydroboration/oxidation reaction on 2-methylbut-2-ene. Upon isolation, Karen runs an optical rotation experiment to determine the enantiopurity of her alcohol. Which of the following statements would be MOST correct regarding the optical rotation of her synthetic sample (Note: the specific rotation of (R)-3-methylbutan-2-ol is -40.4°). O [x] of the sample will be 0° O [x] of the sample will be -40.4° O [x] of the sample will be between 0° and -40.4° O [x] of the sample will be between 0° and +40.4° [x] of the sample will be +40.4°arrow_forward
- b) The TCICA test for secondary and primary alcohols. Draw the structure for alcohol that would quickly give a white precipitate with TCICA test reagent. Write the equation for the reaction using the structure that you drew. The compound should have 15 number of carbons.arrow_forwardOrganic Chemistryarrow_forwardOrganic chemistry student Peter is tasked with synthesizing (R)-3-ethylpentan-2-ol. Using his knowledge of alkene reactions, he runs a hydroboration/oxidation reaction on 3-ethylpent-2-ene. Upon isolation, Peter runs an optical rotation experiment to determine the enantiopurity of his alcohol. Which of the following statements would be MOST correct regarding the optical rotation of his synthetic sample (Note: the specific rotation of (S)-3-ethylpentan-2-ol is +28.2°). O [x] of the sample will be +28.2° O [x] of the sample will be 0° O [x] of the sample will be between 0° and -28.2° O [a] of the sample will be -28.2° O [x] of the sample will be between 0° and +28.2°arrow_forward
- Make a model of cyclobutane and draw its shape. Comment on its bond angles and how easily the model fitted together. Do you think cyclobutane is a stable or an unstable compound? Give reasons to support your answer including diagrams where appropriate.arrow_forwardFractional distillation lab experiment for organic chemistry What are possible other identification methods that can be used to identify the compounds isolated after distillation?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY