
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can someone check my work
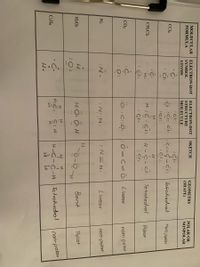
Transcribed Image Text:## Molecular Structures and Properties
This table provides a detailed overview of various molecular structures, their geometries, and polarities.
### Table Columns:
1. **Molecular Formula**
2. **Electrondot Symbol - Atoms**
3. **Electrondot Structure - Molecule Sketch**
4. **Geometry (Shape)**
5. **Polar or Nonpolar**
---
### Details:
1. **C₂H₆ (Ethane)**
- **Electrondot Symbol:**
- H : C : H
- H : C : H
- **Molecule Sketch:**
- H - C - C - H
- | |
- H H
- **Geometry (Shape):** Tetrahedral
- **Polar or Nonpolar:** Nonpolar
2. **H₂O₂ (Hydrogen Peroxide)**
- **Electrondot Symbol:**
- H : O : O : H
- **Molecule Sketch:**
- H - O - O - H
- **Geometry (Shape):** Bent
- **Polar or Nonpolar:** Polar
3. **N₂ (Nitrogen)**
- **Electrondot Symbol:**
- N ≡ N
- **Molecule Sketch:**
- N ≡ N
- **Geometry (Shape):** Linear
- **Polar or Nonpolar:** Nonpolar
4. **CO₂ (Carbon Dioxide)**
- **Electrondot Symbol:**
- O=C=O
- **Molecule Sketch:**
- O=C=O
- **Geometry (Shape):** Linear
- **Polar or Nonpolar:** Nonpolar
5. **CH₂Cl₂ (Dichloromethane)**
- **Electrondot Symbol:**
- H : C : Cl
- H : : Cl
- **Molecule Sketch:**
- Cl
|
H-C-H
|
Cl
- **Geometry (Shape):** Tetrahedral
- **Polar or Nonpolar:** Polar
6. **CCl₄ (Carbon Tetrachloride)**
- **Electrondot Symbol:**
- Cl : C : Cl
- Cl : : Cl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1 of 20 A barrel of crude oil has a volume of 42 gallons, only approximately 45% of which is processed into gasoline. If your car achieves 32 mi/gal, and you drive 36,000 miles in one year, how many barrels of crude oil are required to run your car for a year? Tap here or pull up for additional resourcesarrow_forwardDesign and construct a data table. Be sure to give the table a title.arrow_forwardAnalyze each statement (A and B) whether they are true or false. Use the choices below in answering each item; A statement A is true B statement B is true C both A and B are true D neither A nor B are true (A) A dispersing agent such as Na-hexametaphosphate is needed prior to soil textural analysis. (B) particles greater than 2.0 mm is included in the analysis of soil texture.arrow_forward
- I do need help with this problem. It is multiparty. Thank you!arrow_forwardWhich is more accurate, a transfer pipet or a measuring pipet? How much is the uncertainty in microliters when you deliver (a) 10 !L or (b) 100 !L from a 100- !L adjustable micropipet?arrow_forwardWhat is the “taring” function of the digital electronic balance? Why do you need to “tare” the digital balance before use?arrow_forward
- Home Insert Draw View A Text Mode Lasso Select Insert Space + 4. When measuring the volume of a liquid, how would sample size (e.g., using a 10 mL graduated cylinder vs. a 100 mL graduated cylinder to measure out 70 mL of a liquid) affect the absolute error and percentage error in the measured values of mass and volume and therefore the density?arrow_forwardA bedroom has a volume of 108 m^3. What is the volume in centimeters cubed (cm^3)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY