
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
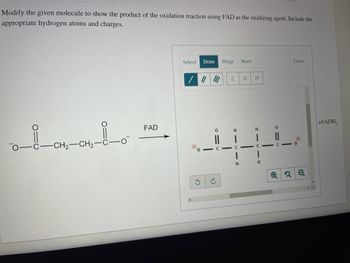
Transcribed Image Text:Modify the given molecule to show the product of the oxidation reaction using FAD as the oxidizing agent. Include the
appropriate hydrogen atoms and charges.
Select Draw Rings More
-CH₂-CH₂-C-0
C
с-
+FADH₂
= |
FAD
0
с-
0
H
H
Erase
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chromic acid oxidation occurs in three steps. Draw the two missing neutral intermediates.arrow_forwardName the first compound on the reactant side of the equation and comment on the way that it is behaving in this reaction.arrow_forwardFigure 5 shows the structures of the inhibitors of acetyl-CoA carboxylase: CABI-CoA and CABI. What structural features of CABI make it a potential inhibitor of acetyl-CoA carboxylase? CoenzymeA-S. NH CABI-COA NH САВI Figure 5arrow_forward
- Step 7 of the citric acid cycle is shown. Which statement best describes what occurs in this step? CO₂ 1 CH || CH + H₂O CO₂ fumarate CO₂™ fumarase HO C-H CH₂ CO₂ malate A) Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase. B) Fumarate undergoes hydration with the aid of the enzyme fumarase. C) Fumarate undergoes hydrolysis with the aid of the enzyme fumarase. D) Fumarate undergoes reduction with the aid of the cofactor fumarase.arrow_forwardPredict the product of each monosaccharide reaction. Modify the molecule to show the product of the reaction. Atoms or bonds may need to be added or removed. 0=0 H-C-OH Н HTC OH H-C-OH H2C-OH 0=0 Н H-C-OH H-C-OH H2C-OH oxidation reduction Reaction A K Select / S С 01 Reaction B Select Draw 1 С Draw / 4 11 C C Rings 0 Н н-с HICOH н — с — Rings More H— с - он Н — он он 0 H нс — он — H- More H-C ОН с-он OH H. C ОН Erase Q2 Q О ▸ Erase 20arrow_forwardQuestion 4 Select the carbon in the following molecule that provides the most energy upon oxidation. OO (Ⓒ) C4 C1 Question 5 HO Identify the inaccuracy in the following equation: NAD +FAD-NADH+H+FADH Use the editor to format your answer 2 CH 2 CH₂ -CH H₂C- OHarrow_forward
- 1. Identify the most likely additional substrates, products, and coen- zymes for each reaction in the following imaginary pathway. COO Н—с—NH— сно CoO COO CH, Н—с—NH—сно H-C-NH3 CH2 CH2 CH2 CH2 H-C-CH3 H-C-CH3 H-C-NH, H-C-NH3 H-C-NH3 COO COO || C-NH2 || C-NH2 C- NH2 H-C-NH3 H-C-NH3 H-C-NH-CH3 CH2 CH2 CH2 H-C-CH3 H-C-CH3 H-C-CH3 H-C- NH3 C=0 C=0 CoO CoO COO -0- O=0- -arrow_forwardFor the following redox reaction: ОН D-beta-hydroxybutyrate dehydrogenase .co .CO .COO H3C H3C acetoacetate NADH + H* NAD + D-beta-hydroxybutyrate Which of the following statements apply? Select all that apply а. NADH is the oxidant b. acetoacetate is the oxidant c. beta-hydroxybutyrate is oxidized O d. beta-hydroxybutyrate is reducedarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY