
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
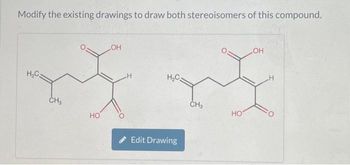
Transcribed Image Text:**Educational Content on Stereoisomers**
---
**Title: Understanding Stereochemistry through Molecular Structures**
**Text:**
Modify the existing drawings to draw both stereoisomers of this compound.
**Diagrams:**
1. **First Diagram:**
- The molecular structure shows a carbon chain with two carboxyl groups (COOH) attached at both ends.
- A hydroxyl group (OH) is attached to the second carbon from the left.
- This configuration illustrates one stereoisomer.
2. **Second Diagram:**
- This structure is similar to the first, with the hydroxyl group (OH) again attached to the second carbon.
- The orientation of the groups is altered to represent a different stereoisomer.
**Instructions:**
- Students are encouraged to interact with the molecular drawing tool using the "Edit Drawing" button.
- Experiment with altering the bonds and orientations to understand the concept of stereoisomerism in organic chemistry.
**Objective:**
Learners will gain a practical understanding of how molecular orientation affects chemical properties and the concept of stereoisomers.
---
(Note: Be cautious to understand spatial arrangements visually when editing or modifying chemical structures.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compounds listed below are CH3 CH3 HO .. .. OH Br H H Br a. identical b. not isomers C. constitutional isomers d. diastereomers e. enantiomersarrow_forwardFor which double bonds are stereoisomers possible?arrow_forwardDraw the product in the box and show the initiation step, first propagation step, and second propagation step.arrow_forward
- Explain why compound A has two stereoisomers but compounds B and C exist as single compounds.arrow_forwardPlease help with the substituentarrow_forwardStudy Guide Questions Q7. Identify each Fischer projection as the D- or L-isomer H H - OH H-OH H-OH H-OH CH2OH HO- H HO CH₂OH C=0 - H OH -H CH₂OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY