
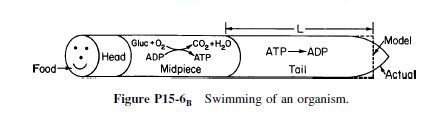
The swimming rate of a small organism [J. Theoret. Biol.,26, 11 (1970)] is related to the energy released by the hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP). The rate of hydrolysis is equal to the rate of diffusion of ATP from the midpiece to the tail (see Figure P15-6
B). The diffusion coefficient of ATP in the midpiece and tail is 3.6 x 10- 6cm2/s. ADP is converted to ATP in the midsection, where its concentration is 4.36x10-5mol/cm3. The cross-sectional area of the tail is 3x10-10cm2.
(a)Derive an equation for diffusion and reaction in the tail.
(b)Derive an equation for the effectiveness factor in the tail.
(c)Taking the reaction in the tail to be of zero order, calculate the length of the tail. The rate of reaction in the tail is 23x10-18mol/s.
(d)Compare your answer with the average tail length of 41µm. What are possible sources of error?
Trending nowThis is a popular solution!
Step by stepSolved in 8 steps with 14 images

- feed back to aid with ques- (a) In this question a suspension is settling over time. As time progresses the volume of the clear layer at the top of the cylinder will increase. You know that the clear layer has a cylindrical shape. Therefore, you can calculate the height of the clear layer at each volume given using the equation for the volume of a cylinder. You are told that you can calculate the single particle velocity at time point 1 which corresponds to the point where the volume of the clear layer is 29 mL. answers Time (s) 0,1.6,5.3, 26.4arrow_forwardThe alkali metals (Li, Na and K) are promising energy storage materials used in batteries. Lithium-ion batteries are found in almost all electronic devices encountered in daily life. The performance of these devices is constrained in part by the transport of alkali metal ions in their electrolytes. Please calcu.ate the diffusion coefficient of Li*, Na* and K* (with a Cl- counterion) by treating them as hard spheres.arrow_forwardA long glass capillary with a diameter of 0.1 cm is in contact with water on one side and dry air on the other. Water vapor evaporates from the side in contact with water and diffuses through the capillary to the other side. Using the following given data, calculate the rate at which water evaporates in this system in [g/s].Isothermal diffusion (20℃)Vapor pressure of water 17.5 mmHg (20℃)Water diffusivity 0.3 cm2/s (20℃)Dry air pressure 760 mmHgDural length 10 cmGas constant R=82 cm3atm/mol K18 g/mol molecular weight of waterarrow_forward
- Consider the problem of how plants might lift water from ground level to their leaves. Assume that there us a semipermeable membrane at the roots, with pure water on the outside, and an ideal solution inside a small cylindrical capillary inside the plant. The solute mole fraction inside the capillary is x = 0.001. The radius of the capillary is 0.1 mm. Assuming the density of the solution = 1 g/mL, what is the height of the solution at 298 K? Can osmotic pressure account for raising this water?arrow_forwardWhat is the fundamental differential equation governing mass transfer in chemical engineering, and how does it relate to Fick's law of diffusion?arrow_forwardA sheet of steel with 100cm2 area has nitrogen(N2) atmospheres on both sides and is permitted to achieve a steady-state diffusion condition. In order to increase the flow rate of N2, which of the following modifications is useful? Steel sheet O Increase the N2 pressure at C1 O Decrease the temperature. O Increase the N2 pressure at C2 O Increase the thickness of the steel sheet.arrow_forward
- Catalyst particles are entrapped in the center of a porous membrane material. The purpose of this is to protect the catalyst so that it can be recovered and reused. This structure shown below is immersed in an aqueous solution containing a proprietary reactant called component A. Component A can only diffuse through the top and bottom faces of this structure. The dimensions of the top face and the bottom face are same (10cm x 10cm). The thickness of the top membrane is 0.1cm and the thickness of the bottom membrane is also 0.1cm. Furthermore component A is instantaneously consumed by the catalyst at a constant rate of 144 mg/hour when it enters the compartment where the catalyst is located. If the effective diffusivity of component A in this application is 2 x 10-5 cm2/s in the membrane calculate the concentration of component A in the surrounding solution that is needed to maintain the consumption rate. (You may assume the surrounding solution is well mixed and thus void of any…arrow_forwardA quiescent body of water has a depth of 600 mm. The DO level at the bottom after 16 days is 3.5 mg/l when the surface is exposed to the atmosphere at a temperature of 25oC. Determine the DO of the water body. The saturated DO at 25oC is 8.5 mg/l and the diffusion coefficient (kd) at 25oC is 2.5 x 10-3 mm2/s.arrow_forwardAn input stream contains oil sands, comprising 14.0% oil, the remainder being solid sand particles. In the initial stages of steady-state processing, the oil sands are mixed with warm water and pumped to a settling tank. In this tank three layers are formed. The three layers are: 1. Bottom layer containing 95.0% sand and 5.00% water 2. Middle layer containing 5.00% oil and 95.0% water 3. Top layer containing 70.0% oil and 30.0% water The top layer is skimmed off and sent for further processing, while the bottom two layers are disposed of. How much warm water is added to 200 tonnes of oil sands in this process if 85.0% of the total oil is recovered in the top layer (3 sig. figs)? All percentages given are weight percentages.arrow_forward
- 1arrow_forwardProblem #1: Diffusion-convection problems wherein the species diffusivity is concentration-dependent often provide a differential equation of the following general form: [vLj dC + [DJ dz d [dc where Cis the dimensionless concentration and z is the dimensionless distance. Assume VLID, is a constant. Assume that C=1 at z = 0, and C=0 when z is very large. Solve the above differential equation and provide an exact analytical equation for C=ft).arrow_forward2. In order to remember the difference between macroscopic and molecular scale energies, I ... Answer:arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





