
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Solve full accurate dont use guideline answer ok
Solve full accurate with diagrams okk.
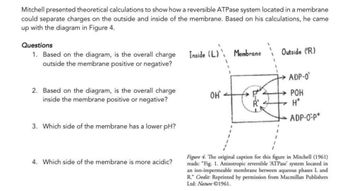
Transcribed Image Text:Mitchell presented theoretical calculations to show how a reversible ATPase system located in a membrane
could separate charges on the outside and inside of the membrane. Based on his calculations, he came
up with the diagram in Figure 4.
Questions
1. Based on the diagram, is the overall charge
outside the membrane positive or negative?
2. Based on the diagram, is the overall charge
inside the membrane positive or negative?
3. Which side of the membrane has a lower pH?
4. Which side of the membrane is more acidic?
Inside (L) Membrane
ОН'
Outside (R)
ADP-0
POH
H*
ADP-O-P*
Figure 4. The original caption for this figure in Mitchell (1961)
reads: "Fig. 1. Anisotropic reversible ATPase' system located in
an ion-impermeable membrane between aqueous phases L and
R." Credit: Reprinted by permission from Macmillan Publishers
Ltd: Nature 1961.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- This is a part of a review I'm studying, NOT a graded assignment, please do not reject.arrow_forwardWhat do you think the additives do? What effect will they have? answerarrow_forwardearn-us-east-1-prod-fleet01-xythos.learn.cloudflare.blackboardedn.com/blackboard.learn.xythos.prod/5a333e152baa2/10422334?X-Bla.. to 3 YtMp3 ) YouTube to MP3 Co.. O A Sublime Trance(S.. O Kajri U. P. Folk Dhu... O Kajri : Dadra Taal (S. OD Insurance Policie... M Gmail YouTube 7 /11 175% 1. On the diagram below indicate three virulence factors and describe how they aid the bacteria in causing an infection. chromosome (nucleoid region) pili ribosomes, food granule prokaryotic flagellum capsule or slime layer 'cell wall cytosol plasma membrane plasmid (DNA) 05/0 DELLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON