
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
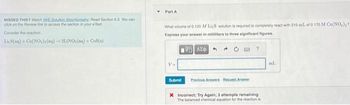
Transcribed Image Text:MISSED THIS? Watch (WE Solution Stichiometry: Read Section 5.3. You can
click on the Review link to access the section in your Text
Consider the reaction
LisS(aq) + Co(NO₂)(aq) + 2LINO, (aq) + Cos(e)
Part A
What volume of 0.120 M LisS solution is required to completely react with 215 ml of 0.170 M Co(NO₂),?
Express your answer in milliliters to three significant figures.
VAX+
Submit
+
Previous Answers Request Answer
X Incorrect; Try Again; 3 attempts remaining
The balanced chemical equation for the reaction is
ml.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 13 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Silver ion (Ag) can be analyzed by titration with chloride ion. The reaction (ionic form) involved is Ag(aq)+Cl^ - (aq)—>AgCl(s) If a 0.2150 g sample silver alloy required 25.70 mL of 0.05410 M NaCl to reach the end point, what is the percent silver in the alloy sample?arrow_forward3. Why is it proper to read the initial volume of the buret instead of trying to adjust the volume to a round number such as 0 mL? 4. Calculate the molarity of a NaOH solution if 23.25 mL of NaOH is titrated with 0.475 grams of KHP and 1.25 mL of 0.100 M HCI is required.arrow_forwardIn the laboratory a student combines 47.3 mL of a 0.247 M aluminum iodidesolution with 11.4 mL of a 0.431 M aluminum acetate solution. What is the final concentration of aluminum cation ?arrow_forward
- 8. A student adds 22.36 mL of 1.366 x 10¹ M Mg(OH), in order to completely neutralize 25.00 mL of H₂PO acid. Calculate the original concentration of the acid solution.arrow_forwardWhat volume in mL of an 11.6 M HCIO, (100.5 g/mol) solution is needed to make 500.0 mL of a 7.59 M HCIO4 solution? Do not put units in your answer. Report your answer as a whole number (no decimal places).arrow_forwardcomplete part darrow_forward
- An aqueous solution of perchloric acid is standardized by titration with a 0.187 M solution of barium hydroxide. If 25.2 mL of base are required to neutralize 22.6 mL of the acid, what is the molarity of the perchloric acid solution? M perchloric acidarrow_forwardConsider the neutralization reaction 2 HNO3(aq) + Ba(OH)2(aq). 2 H2O(l) + Ba(NO3)2(aq) A 0.105 L sample of an unknown HNO3 solution required 42.5 mL of 0.250 M Ba(OH)2 for complete neutralization. Wha the concentration of the HNO3 solution? concentration: .098arrow_forwardAn aqueous solution of barium hydroxide is standardized by titration with a 0.122 M solution of hydrobromic acid. If 15.4 mL of base are required to neutralize 16.8 mL of the acid, what is the molarity of the barium hydroxide solution? M barium hydroxidearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY