
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Help with part B in particular, thank you
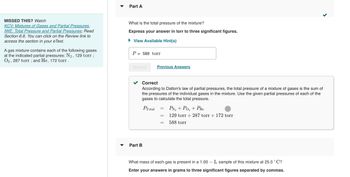
Transcribed Image Text:MISSED THIS? Watch
KCV: Mixtures of Gases and Partial Pressures,
IWE: Total Pressure and Partial Pressures; Read
Section 6.6. You can click on the Review link to
access the section in your eText.
A gas mixture contains each of the following gases
at the indicated partial pressures: N₂, 129 torr;
O2, 287 torr; and He, 172 torr.
Part A
What is the total pressure of the mixture?
Express your answer in torr to three significant figures.
► View Available Hint(s)
P
=
588 torr
Submit
Part B
Previous Answers
Correct
According to Dalton's law of partial pressures, the total pressure of a mixture of gases is the sum of
the pressures of the individual gases in the mixture. Use the given partial pressures of each of the
gases to calculate the total pressure.
PTotal
=
=
=
PN₂ + PO₂ + PHe
129 torr + 287 torr + 172 torr
588 torr
<
What mass of each gas is present in a 1.00 - L sample of this mixture at 25.0 °C?
Enter your answers in grams to three significant figures separated by commas.
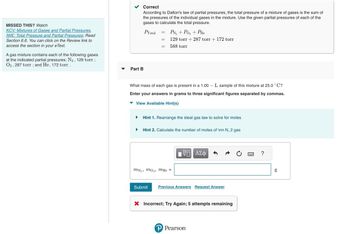
Transcribed Image Text:MISSED THIS? Watch
KCV: Mixtures of Gases and Partial Pressures,
IWE: Total Pressure and Partial Pressures; Read
Section 6.6. You can click on the Review link to
access the section in your e Text.
A gas mixture contains each of the following gases
at the indicated partial pressures: N₂, 129 torr;
O2, 287 torr; and He, 172 torr.
Correct
According to Dalton's law of partial pressures, the total pressure of a mixture of gases is the sum of
the pressures of the individual gases in the mixture. Use the given partial pressures of each of the
gases to calculate the total pressure.
PTotal
Part B
=
=
=
MN 29
PN₂ + PO₂ + PHe
129 torr + 287 torr + 172 torr
588 torr
What mass of each gas is present in a 1.00L sample of this mixture at 25.0 °C?
Enter your answers in grams to three significant figures separated by commas.
View Available Hint(s)
Hint 1. Rearrange the ideal gas law to solve for moles
Hint 2. Calculate the number of moles of \rm N_2 gas
mo₂, mHe =
|VD ΑΣΦ
Submit Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
P Pearson
8.0
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What amide(s) would form the following amine (shown below) upon treatment with Li AIH? Note: You can upload your image to this problem OR you can upload one document with your work for ALL problems to the question at the END of this exam. pdf, doc, docx, png, jpg, jpeg, tiff, gif files only! ↑ Drag n' Drop here or Browsearrow_forwardOrganic Functional Groups Identifying positions labeled with Greek letters in acids and derivatives If possible, replace an H atom on the a carbon of the molecule in the drawing area with a methyl group substituent, and replace an H atom on the ß carbon with a hydroxyl group substituent. If one of the substituents can't be added for any reason, just don't add it. If neither substituent can be added, check the box under the drawing area. HO Oneither substituent can be added. OH 0/5 Xarrow_forwardWhich pesticide doesn't have a halogen functional group?ImazapyrNitisinone2,4,5-Trichlorophenoxyacetic acidSimazineAll of these have a halogenarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardFix each molecule in the drawing area below so that it has the structure it would have if it were dissolved in a 0.1 (aq) solution of NaOH 3-hydroxypropanoic acid and 1,3-dihydroxy-2-propanone .arrow_forwardShow mecarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY