
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
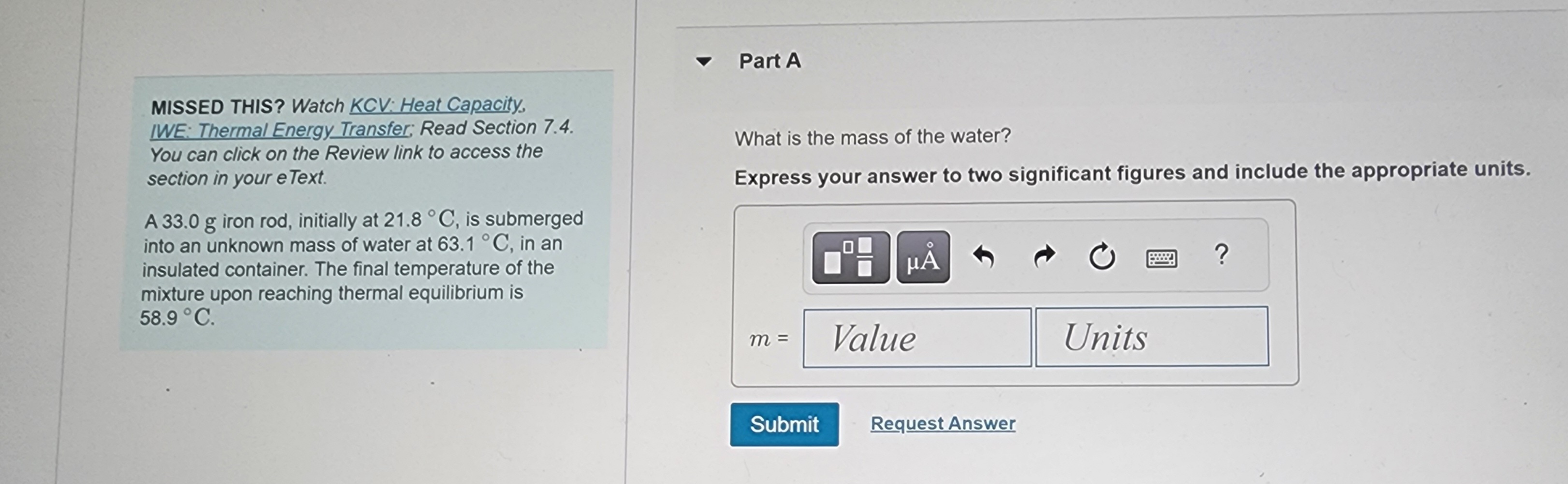
Transcribed Image Text:MISSED THIS? Watch KCV: Heat Capacity,
IWE: Thermal Energy Transfer; Read Section 7.4.
You can click on the Review link to access the
section in your e Text.
A 33.0 g iron rod, initially at 21.8 °C, is submerged
into an unknown mass of water at 63.1 °C, in an
insulated container. The final temperature of the
mixture upon reaching thermal equilibrium is
58.9 °C.
Part A
What is the mass of the water?
Express your answer to two significant figures and include the appropriate units.
m =
Submit
P9 HÅ
Value
Request Answer
Units
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- To treat a burn on his hand, a person decides to place an ice cube on the burned skin. The mass of the ice cube is 15.0 g, and its initial temperature is –11.2 °C. The water resulting from the melted ice reaches the temperature of his skin, 29.2 °C. How much heat is absorbed by the ice cube and resulting water? Assume that all of the water remains in the hand. Constants for water can be found in this table.arrow_forwardA hot 105.9 g lump of an unknown substance initially at 154.0 °C is placed in 35.0 mL of water initially at 25.0 °C and the system is allowed to reach thermal equilibrium. The final temperature of the system is 36.2 °C. Using this information and the specific heat values for several metals in the table, identify the unknown substance. Assume no heat is lost to the surroundings. rhodium ○ graphite titanium zinc tungsten aluminum Substance Specific heat (J/(g∙°C)) aluminum 0.897 graphite 0.709 rhodium 0.243 titanium 0.523 tungsten 0.132 zinc 0.388 water 4.184arrow_forwardA 2.5 g sample of french fries is placed in a calorimeter with 500.0 mL of water. The initial temperature of the water is 21.0 0C. After combustion of the french fries, the water has a temperature of 48.0 0C. Calculate the heat gained by the water (calories). Remember--the density of water is 1.00 g/mL. Report your answer to three significant figuresarrow_forward
- A 27-gram piece of metal at 144 oC is dropped into 86 grams of water at 29 oC. The water temperature rose to 37 oC. Calculate the heat change of the metal in Joules. (DO NOT PUT UNITS IN YOUR ANSWER.) Assume that all of the heat lost by the metal is transferred to the water and no heat is lost to the surroundings. The specific heat of water is 4.184 J / g oC.arrow_forwardIn a simple calorimeter, a piece of metal weighing 48.67 g was heated to 120.0 °C and then put it into 100.0 mL of water (initially at 23.7 °C). The metal and water were allowed to come to an equilibrium temperature, determined to be 27.8 °C. Assuming no heat lost to the environment, calculate the specific heat of the metal. Is this process endothermic or exothermic? Explain. Show Your Workarrow_forwardPlease help me complete this questionarrow_forward
- (please type answer no write by hand)arrow_forwardMISSED THIS? Watch KCV: Heat Capacity, IWE: Thermal Energy Transfer; Read Section 7.4. You can click on the Review link to access the section in your e Text. A 32.1 g iron rod, initially at 21.5 °C, is submerged into an unknown mass of water at 63.5 °C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 58.5 °C. Part A What is the mass of the water? Express your answer to two significant figures and include the appropriate units. m = Submit 0 °H | |µÅ Value Units Previous Answers Request Answer ?arrow_forwardA chemist burned a sample of coal in a bomb calorimeter. Her results are below. Calorimeter's Heat Capacity 7.264 kJ/°C Mass of Coal Initial Temperature Final Temperature 0.100 g 22.07°C 25.37°C The sample of coal that the chemist placed in the bomb was dry but when she finished the test there was a small amount of water in the bomb. Where did this water come from and how does it affect her result? The water came from the water surrounding the bomb so her measured heat of combustion is for a closed system. The water was a product of combustion so her measured heat of combustion is for an open system. The water came from the water surrounding the bomb so her measured heat of combustion is for an open system. The water was a product of combustion so her measured heat of combustion is for a closed system.arrow_forward
- 1. A thermometer placed in a solution undergoing a chemical reaction indicates an increase in temperature as the reaction proceeds. Is this reaction endothermic or exothermic? Describe if heat energy is lost or gained from the reaction (the system) to the surroundings. What is the sign of the enthalpy change (AH) of this reaction? 2. A student performs a reaction and determines the enthalpy change (AH) to be 31.4 kJ. Will the temperature of the surrounding solution increase or decrease as a result of this chemical process? 3. If you hold 3 grams of ice in your hand at room temperature, your hand will become cold. a) Is the reaction H,O(s) – H,O(1) endothermic or exothermic? b) In which direction does heat flow?arrow_forwardIn order to break water into hydrogen and oxygen, water is heated to more than 500°C. Which kind of reaction is this and why? It is exothermic because heat needs to be released by the reactants to form the products. It is endothermic because heat needs to be released by the reactants to form the products It is exothermic because heat needs to be absorbed by the reactants to form the products. It is endothermic because heat needs to be absorbed by the reactants to form the products.arrow_forwardA metal object with mass of 21.4 g is heated to 97.0 °C and then transferred to an insulated container containing 81.7 g of water at 20.5°C. The water temperature rises and the temperature of the metal object falls until they both reach the same final temperature of 23.1 °C. What is the specific heat of this metal object? Assume that all the heat lost by the metal object is absorbed by the water. cal specific heat:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY