
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
![### Mole Concept and Mass Calculation
#### Part B:
**32.9 mmol Cl₂**
**Problem Statement:**
Express your answer to three significant figures and include the appropriate units.
**Calculate the mass in grams (g) of each sample:**
**Formula:**
\[ \text{m(Cl₂)} = \text{Value in } \, \text{Units} \]
**Input Fields:**
- **Value** (input your calculated value here)
- **Units** (specify the units of your answer here)
**Action Buttons:**
- **Submit** - Click to submit your answer.
- **Request Answer** - Click to get assistance on the problem.
---
##### Recommended Study Resources:
**If you're struggling with this problem, watch the following instructional video:**
[**IWE: The Mole Concept—Converting Between Mass and Number of Molecules**](#)
Additionally, refer to **Section 3.8** in your textbook. You can click on the Review link to access this section in your eText.
**Note:** Understanding the mole concept and how to convert between mass and the number of molecules is crucial for solving this problem.
---](https://content.bartleby.com/qna-images/question/6ae95cc5-a0cd-43b0-8331-9678c9dacca0/b1ed1430-95cf-46dd-a595-a5b7841bfb06/lxq2bq.jpeg)
Transcribed Image Text:### Mole Concept and Mass Calculation
#### Part B:
**32.9 mmol Cl₂**
**Problem Statement:**
Express your answer to three significant figures and include the appropriate units.
**Calculate the mass in grams (g) of each sample:**
**Formula:**
\[ \text{m(Cl₂)} = \text{Value in } \, \text{Units} \]
**Input Fields:**
- **Value** (input your calculated value here)
- **Units** (specify the units of your answer here)
**Action Buttons:**
- **Submit** - Click to submit your answer.
- **Request Answer** - Click to get assistance on the problem.
---
##### Recommended Study Resources:
**If you're struggling with this problem, watch the following instructional video:**
[**IWE: The Mole Concept—Converting Between Mass and Number of Molecules**](#)
Additionally, refer to **Section 3.8** in your textbook. You can click on the Review link to access this section in your eText.
**Note:** Understanding the mole concept and how to convert between mass and the number of molecules is crucial for solving this problem.
---
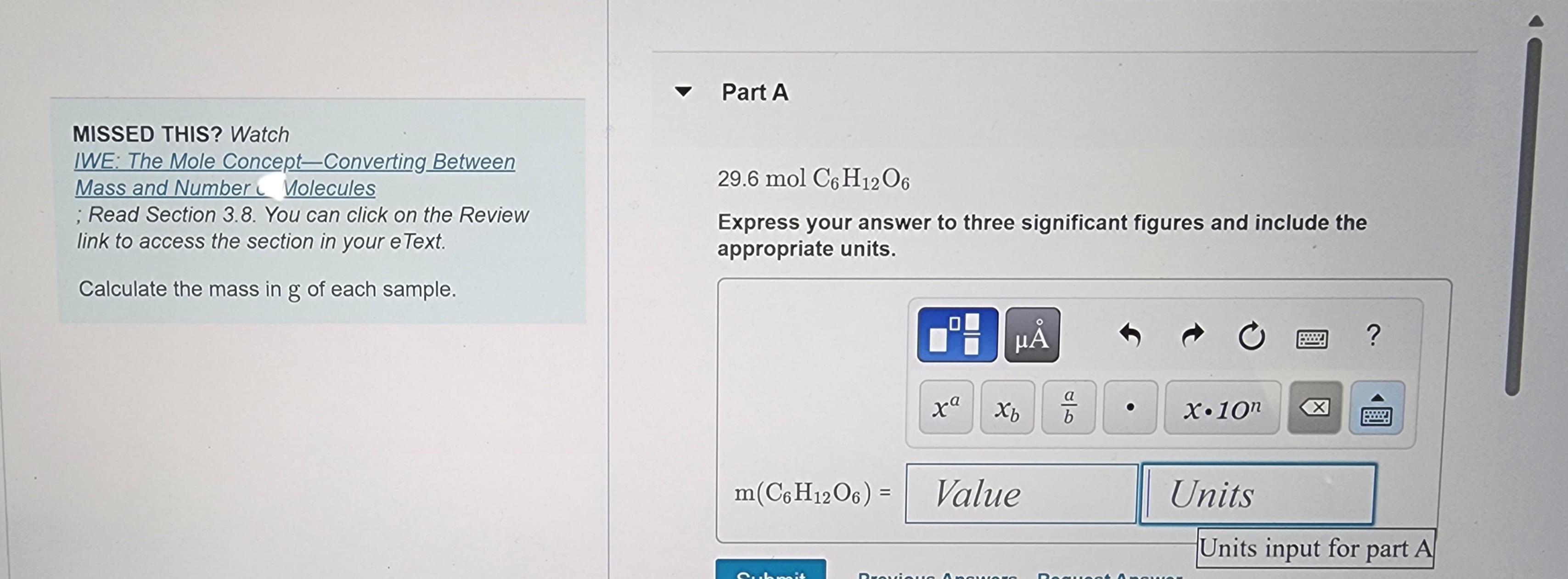
Transcribed Image Text:MISSED THIS? Watch
IWE: The Mole Concept-Converting Between
Mass and Number Molecules
; Read Section 3.8. You can click on the Review
link to access the section in your e Text.
Calculate the mass in g of each sample.
Part A
29.6 mol C6H12O6
Express your answer to three significant figures and include the
appropriate units.
xa
Submit
μÅ
Xb
m(C6H12O6) = Value
Previous Answer
b
Reque
Ć
X.10n
Units
X
?
Units input for part A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student in lab needs to make a solution that is 4.00% by mass NaCl. If 165 g of NaCl is available, what mass of solution can be prepared? mass of solution=arrow_forwardAnswer this question in the same format thats in the picture below on paper, answer correctly.Question: how many grams are in 1.73 mol of dinitrogen pentoxide (N2O5)arrow_forwardA student prepared benzil according to the experimental procedure using 2.58 g. of benzoin and 12 mL of nitric acid. The mass of the crude benzil product was 2.25 g. and the mass of the recrystallized benzil product was 1.95 g. Find the molecular weights for benzoin and benzil, then answer the following questions. What was the percent yield of the crude benzil? Assume units of %. Round answer to the first decimal place. What was the percent yield of the purified benzil? Assume units of % Round answer to the first decimal place. What was the percent recovery of the purified benzil from the crude benzil? Assume units of % Round answer to the first decimal place.arrow_forward
- calculate the mass of calcium carbonate in the weighing boat using the information below. Mass of weighing boat 2.26 g Mass of calcium carbonate & weighing boat 9.80 g Mass of calcium carbonate _____ g For Part B, calculate the number of moles of calcium carbonate using a molar mass of 100.09 g/mol for calcium carbonate. Label each of your 2 answers when you type your response.arrow_forwardUsing the numbers below how do I set this up to get the correct answer?arrow_forward3. Stoichiometry Calculations - 1, 2, or 3 step B's Mass, Mass, Use: 2 C,H, (g) + 7 0, (g) → 4 Co, (g) + 6 H,0 (g) Molar Mass of B Molar Mass of A Mole - Mole Use Coefficients Complete with dimensional analysis. Show the answer with units. B's Mole A's Mole 1. Find the mass in grams of C,H; that reacts with 5.60 moles of 02. Given amount Answer Work (by dimensional analysis) and units with units 2. When 6.0 grams of C2H, is used then how many moles of CO2 are formed? Given amount Answer Work (by dimensional analysis) and units with units 3. Find the mass of H,0 gas formed by the reaction of 9.6 g 02. Given amount Answer Work (by dimensional analysis) and units with unitsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY