
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
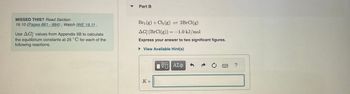
Transcribed Image Text:MISSED THIS? Read Section
19.10 (Pages 881 - 884); Watch IWE 19.11.
Use AG values from Appendix IIB to calculate
the equilibrium constants at 25°C for each of the
following reactions.
-
Part B
Br₂(g) + Cl₂ (g) = 2BrCl(g)
AG (BrCl(g)) = -1.0 kJ/mol
Express your answer to two significant figures.
► View Available Hint(s)
K =
—| ΑΣΦ
C
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The table below shows the equilibrium constants of a reaction at varying temperatures (measure in °C) and is the same data shown in Question #1 for the reaction A B T (°C) -50.5 -25.1 0.5 25.2 51.2 75.2 100.2 125.5 150.4 Using the data from the table, calculate the change in entropy (DS) in unit of J/mol*K for this reaction. Input your answer with 2 significant figures. K 100 116 130 143 156 167 177 187 196arrow_forwardThe equilibrium constant, Keq, for the reaction 2 HI (g) = H2 (9) + 12 (g) is 1.14 x 10-3 at 25 °C. Calculate AG* for this reaction. O 1.53 x 10-3 kJ -37.8 kJ O 6.79 kJ 16.8 kJ O-76.1 kJarrow_forwardUse the values given in Appendix L in your textbook to calculate K. for the reaction between Fe2+(aq) and Zn(s) at 25 °C. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answerarrow_forward
- For the reaction below, the AH° = -113.1 kJ/mol and AS = -145.3 J/K-mol. Calculate the equilibrium constant, K, for this reaction at 25°C. 2NO(g) + O2(g) 2NO2(g)arrow_forwardGiven the following information below, determine AGxn at equilibrium. 12(g) + Cl2(g) 2 ICI(g) 11 K, = 81.9 (at 298 K) O 1 O +10.9 kJ O -10.9 kJarrow_forwardChp 19 #28 & # 30arrow_forward
- I want solutions of all parts... Otherwise I will downvotearrow_forward16arrow_forwardCalculate the AH°, AS°, and AG° at 298 K for the following reaction: (Use the values found in Thermodynamic Properties to calculate your answers for questions a and b.) Al2O3(s) + 3 C(graphite) + 3 Cl₂(g) → 2 AICI 3(s) + 3 CO(g) 4.0 -64 a) ΔΗΟ kJ/mol b) AS° ✓ J/K.mol kJ/mol (Determine from the temperature, AH and AS) c) AGO 4.0 76.9 4.0 -89 (d) Is the reaction extensive at standard conditions and 298 K? O Yes O NO (e) Write the expression for K. (Format example: Kp = pHC12 / pH₂. pCl₂ would be entered as K_{p} = pHCI^{2}/pH_{2}. pCl_{2}.) Help chemPad XX (PCO)3 (pCl₂)³ ← Greek (PCO)^3/(pCl_2)^3 Your answer provides a different type of equation than was expected. (f) Calculate the value of K at 298 K 2.51e-16 X (Please answer to 3 significant figures.) (g) Estimate the value of K at 600 K. 1.79e-8 X (Please answer to 3 significant figures.)arrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH° = -111 kJ/mol, and K. = 0.159 , at 723 K. N2 (g) + 3 H, (g)=2 NH3 (g) When 0.21 moles of NH, (g) are added to the equilibrium system at constant temperature: The value of K The value of Qe K. The reaction must O run in the forward direction to restablish cquilibrium. O run in the reverse direction to restablish coquilibrium. O remain the same. It is already at cquilibrium. The concentration of H, will Submit Answer Retry Entire Group 1 more group attempt remainingarrow_forward[Review Topics] [References) Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH° =-10.4 kJ/mol, and K. = 55.6 , at 698 K. H2 (g) + I2 (g)=2 HI (g) When 0.26 moles of HI (g) are added to the equilibrium system at constant temperature: The value of K. The value of Q. v Ke The reaction must Orun in the forward direction to restablish equilibrium. Orun in the reverse direction to restablish equilibrium. O remain the same. It is already at equilibrium. The concentration of I, will Submit Answer Retry Entire Group 9 more group attempts remaining (Previ ins prt sc delete fg f10 f12arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY