
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Enter a chemical equation for the second ionization step of arsenic acid.
Express your answer as a chemical equation including phases.
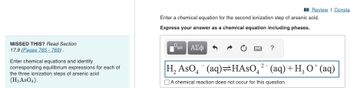
Transcribed Image Text:MISSED THIS? Read Section
17.9 (Pages 765 - 769).
Enter chemical equations and identify
corresponding equilibrium expressions for each of
the three ionization steps of arsenic acid
(H3 AsO4).
Enter a chemical equation for the second ionization step of arsenic acid.
Express your answer as a chemical equation including phases.
ΑΣΦ
2
Review | Consta
?
H₂ AsO4 (aq)=HASO² (aq) + H₂ O* (aq)
A chemical reaction does not occur for this question.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please write a balanced equations and indicate the state. HCl(aq) + NH4Clarrow_forwardWhich of the following statements is true? Insoluble ionic compounds are completely insoluble. All chemical reactions proceed to completion, forming 100% yields of products. Nonspontaneous reactions favor reactants at equilibrium. No chemical reaction is occurring, once no further changes can be observed. All of the above statements are false.arrow_forwardWrite the formula for a micelle of a sol of barium sulfate, obtained by the exchange reaction between barium nitrate and potassium sulfate in the case of an excess of barium nitrate.arrow_forward
- Enter a molecular equation for the reaction of dilute sulfuric acid with iron. (Assume that sulfuric acid acts as a diprotic acid and that an iron(II) compound is formed.) Express your answer as a balanced chemical equation. Identify all of the phases in your answer. Enter a molecular equation for the reaction of hydrobromic acid with magnesium. Express your answer as a balanced chemical equation. Identify all of the phases in your answer. Enter a molecular equation for the reaction of acetic acid, CH3COOHCH3COOH, with zinc. Express your answer as a balanced chemical equation. Identify all of the phases in your answer.arrow_forwardlook at the dissolution of solid sodium chloride in water. In answering the following questions, you may draw, but not replace, your explanation with the drawing a) Describe the structural features and type of chemical bonding that exists in solid sodium chloride. b) Describe the structural features and the type of chemical bonding that exist in water molecules. c) Explain how water molecules dissolve salts like sodium chloride.arrow_forwardThe pH of an aqueous solution of acetic acid (CH,COOH) is 2.5229. What is the initial molar concentration of HCOOH, if its acid ionization constant is K, = 1.8x10°? Enter a your answer in the box provided with correct units and sig. figs.: The initial molar [CH,COOH], = Answer: concentration of : %3D acetic acid isarrow_forward
- Part B Balance each of the following neutralization reactions. H2SO4(aq)+Al(OH)3(s)→H2O(l)+Al2(SO4)3(aq) Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forwardFor Cr3+, enter an equation that shows how the cation acts as an acid. Express your answer as a chemical equation including phases.arrow_forwardChemistry Br OH OH Pd catalyst, base (d) "Den Br OR OR Pd catalyst, basearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY