
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
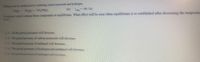
Transcribed Image Text:Methanol can be synthesized by combining carbon monoxide and hydrogen.
AH
Cog) + 2H2(g) CH3OH(g)
A reaction vessel contains these compounds at equilibrium. What effect will be seen when equilibrium is re-established after decreasing the temperatu
IXn=-90.71J
45°C?
OA. All the partial pressures will decrease.
OB. The partial pressure of carbon monoxide will decrease.
OC. The partial pressure of methanol will decrease.
OD. The partial pressures of hydrogen and methanol will decrease.
O E. The partial pressure of hydrogen will increase.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction system Fe(s) + H2 O(g)= FeO(s) + H2 (9) which has already reached a state of equilibrium, predict the effect that each of the following changes will have on the position of the equilibrium. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected. a The pressure of hydrogen is increased by injecting an additional mole of hydrogen gas into the reaction vessel. shifts left shifts right no effect b Hydrogen gas is removed as it forms by use of a chemical absorbent, or "scrubber." shifts left shifts right no effect C An additional amount of solid iron is added to the reaction vessel. shifts left shifts right no effectarrow_forwards ppe (p) the eats of reaction. heactants 2. Write the equilibrium expression for each of the following reactions (a) 2HB1(g) → H2(g) + Br2(g) (b) CH4(g) + C2(g) → CH;Cl(g) + HCl(g)arrow_forwardFor the reaction at equilibrium: 2 H2O (g) +2 Cl 2 (g) + energy4 HCI (g) + O2 (g), If the temperature in the reaction vessel is decreased: O [H2O] will decrease. [H2O] will increase. [H2O] will not change.arrow_forward
- Consider the following reaction at equilibrium. What effect will doubling the temperature have on the system? CH4 ( + 2 02 () = CO2 (g) + 2 H2O () AH° = -882.0 kJ O No shift will occur because the volume of the system has not changed. No shift will occur because the total number of gas molecules on both sides of the chemical equation are equal. O A shift left towards reactants will occur. O No shift will occur because none of the concentrations have changed. O A shift right towards products will occur.arrow_forward3.) For the following equilibrium reaction2NO2(g) ↔N2O4(g) + Δlook in the Tro text to see what LeChatelier's principle states about gases. Assume that the reaction occurs in an enclosed movable piston. [Hint: remember that PV=nRT] a.) The pressure of the reaction is increased. b.) The volume of the reaction is decreased. c.) The volume of the reaction is increased.arrow_forward■ W Chapter... G N. O LCG a Ⓒ BA H H The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium. (The system is considered in equilibrium if K, and Q are within 5% of each other.) (a) 2 NH3(g) = N₂(g) + 3 H₂(g) [NH3] = 0.485 M, [N₂]; = 0.160 M, [H₂] = 0.110 M, K = 18 What is the reaction quotient? What direction will the reaction shift to? O To the left, i.e. the reactant-side. O To the right, i.e. the product-side. O It will not shift. (b) 2 NH3(9) N₂(g) + 3 H₂(g) What is the reaction quotient? What direction will the reaction shift to? O To the left, i.e. the reactant-side. P (NH3) = 1.90 atm, P(N₂) = 11.65 atm, P(H₂) = 11.65 atm, K = 6.6x104 O To the right, i.e. the product-side. O It will not shift. (c) 2 SO 3(g) 2 SO₂(g) + O₂(9) What is the reaction quotient? webassign.net [SO3] = 1.65 M, [SO₂), = 1.65 M, [0₂], =…arrow_forward
- Equilibrium: 7. Write the equilibrium expression, K, for the following reactions: 2NH, (g) + CO2 (g) N,CH,0 (s) + H,0 (g) K= N2 (g) + 02 (g) 2 NO (g) K= 8. Consider the following reaction at equilibrium. 2S0, (g) 2S0, (g) + 02 (g) Predict the affect on equilibrium (will it shift left, shift right or no change?): a. Oxygen gas is removed: b. The volume is increased: c. The pressure is increased: d. SO, is added: e. SO, is added:arrow_forwardConsider the following equilibrium: 2NO(g) + Cl₂ (g) 2NOCI (g) AG=-41. kJ Now suppose a reaction vessel is filled with 1.55 atm of chlorine (C1₂). about this system: T Under these conditions, will the pressure of NOCI tend to rise or fall? Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOC1 will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. and 4.77 atm of nitrosyl chloride (NOCI) at 683. °C. Answer the following questions Orise Ofall yes Ono atm O x10 X Sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY