
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please write each step if you can and neatly!
Thanks so much!
Transcribed Image Text:Pearson Sign In
Hayden-McNeil Login e U.S. History - U.S._Hist... 01 Chem101: Transform
Tempo-Markings-BF
Question 6 of 16
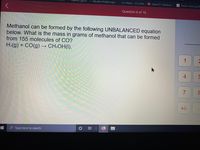
Methanol can be formed by the following UNBALANCED equation
below. What is the mass in grams of methanol that can be formed
from 155 molecules of CO?
H2(g) + CO(g) → CH:OH(I).
1
4
+/-
耳 ||
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 20 MgBr +0=C=O Ⓡ product HCLH₂O → ??arrow_forwardhelp #20 There are two parts, continue with your answer adding PhCH2Brarrow_forward7. You can see an MSDS below. Please answer the following questions related to the MSDS. a) What is the name of this chemical? b) What should you do if someone drinks the chemical? c) Would this chemical catch on fire if it was exposed to flames? d) If this chemical gets in your eye what should you do? e) What color is this chemical? f) What should you do if someone spills a small amount of the chemical?arrow_forward
- Determine the structures of the missing organic molecules in the following reaction: Y II OH X + H₂O H+ H* OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. C X :0 Ś m c+arrow_forwardон ㅅ ·Br OH Multistep synthesisarrow_forwardopy X ols Extensions Help text A % 5 Calibri Et f6 [] 12 D C D 6 f7 IN IUA Page 3: Damage from Industrial Chemicals: Questions & U | 2. Describe how forests can be harvested in a sustainable manner. 7 D 3. Identify two nonrenewable and two renewable resources used to generate electricity. Questions 1. Most plastics are made using chemicals extracted from oil. Currently, only a small fraction of plastic waste is recycled. Describe at least two ways in which an improvement in plastics recycling benefits the environment. fg 8 110 5. Why are open-pit mines so difficult to remediate once they are no longer in operation? 4. How does fracking differ from conventional methods of extracting oil and natural gas from the ground? BADEVES NO Sara Patr Patente avanta AGAVANA ( CI Daphne Moore W hp f10 D insert 9 f11 - te -- E ☆ 0 ATER ANDEREN TOE 10 6 EINE HE CANNES DETE LA STATIONAL CHA MAPANSION MANTAR AN F12 2 DENTAL SERVER SAN AUMENTARE MENTERIAN KE BERSAMA SEMANA to at 247 7 RESTATGE FACE SEE…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY