
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
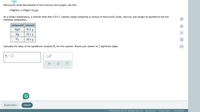
Transcribed Image Text:Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2 HgO(s)→2 Hg(1)+O,(g)
At a certain temperature, a chemist finds that a 8.6 L reaction vessel containing a mixture of mercury(II) oxide, mercury, and oxygen at equilibrium has the
following composition:
compound amount
olo
HgO
20.3 g
Ar
Hg
10.1 g
O2
20.3 g
Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
K = 0
x10
?
G
Explanation
Check
© 2022 McGraw Hill LLC. All Rights Reserved.
Terms of Use | Privacy Center | Accessibility
....................................
.......................................
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following equilibrium reaction taking place inside of a closed 3.0 L container: N2(g) + 3H2(g) = 2 NH3(g) Initially there are 4.5 mol/L of nitrogen gas and 10.4 mol/L of hydrogen gas. When the system reaches equilibrium, there are 3.1 mol/L of nitrogen gas remaining. What is the final concentration of hydrogen and ammonia present in the container?arrow_forwardFor the equilibrium reaction 2SO 2(g) + O 2(g) <-> 2SO 3(g), ΔHº rxn = –198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase? Add SO2 gas. Add a catalyst. None of these. Decrease the temperature. Remove O2 gas.arrow_forwardSuppose a 250. mL flask is filled with 2.0 mol of NO and 1.0 mol of NO₂. The following reaction becomes possible: NO₂(g)+NO(g) — 2NO₂(g) The equilibrium constant K for this reaction is 2.73 at the temperature of the flask. Calculate the equilibrium molarity of NO3. Round your answer to two decimal places. M Sarrow_forward
- For the equilibrium: 3NO2(g) ⇆ N2O5(g) + NO(g) Keq= 1.0 x 10 -11. If a 4.00 L container initially holds 0.20 mol of NO2, how many moles of N2O5 will be present when this system reaches equilibrium?arrow_forwardThe equilibrium system between nitrogen gas, oxygen gas, and nitrogen dioxide gas is given. N2(g)+2O2(g)↽−−⇀ 2NO2(g) Write the balanced chemical equation for the reverse reaction. Include physical states for all species.arrow_forwardSuppose a 500. mL flask is filled with 0.80 mol of NO and 1.8 mol of NO2. The following reaction becomes possible: NO3(g) + NO(g) - 2NO2(g) The equilibrium constant K for this reaction is 4.37 at the temperature of the flask. Calculate the equilibrium molarity of NO. Round your answer to two decimal places.arrow_forward
- The equilibrium system between nitrogen gas, oxygen gas, and nitrogen dioxide gas is given. N2(g)+2O2(g)↽−−⇀ 2NO2(g)N2(g)+2O2(g)↽−−⇀ 2NO2(g) Write the balanced chemical equation for the reverse reaction. Include physical states for all species. chemical equation:arrow_forwardConsider the equilibrium system described by the chemical reaction below. A mixture of gas containing only N2 and H2 is reacted in a vessel at high temperature. At equilibrium, the 5.0 M H2, 8.0 M N2, and 4.0 M NH3 are present. Determine the initial concentrations of H2 and N2 that were present in the vessel. = N2(g) +3 H2(g) 2 NH3(g) 1 Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. Initial (M) Change (M) Equilibrium (M) N2(g) + 3 H2(g) 2 NH3(g) RESET 0 5.0 8.0 4.0 -4.0 10.0 11.0 -2.0 2.0 -5.0 6.0 -6.0 MAR 22 F2 80 F3 F4 1775 % F5 ག|: MacBook Air 6 < 1 F6 27 & tvill AQ বর F7 E R T Y U Ꮴ $ 54 #3 43 S D F G H DII N&S F8 DD F9 D F10 I' 8 * ∞ ) ) 9 0 0 P J K Larrow_forwardSuppose a 250. mL flask is filled with 0.90 mol of N, and 1.2 mol of NH2. This reaction becomes possible: ME N,(g) +3H,(g) – 2NH, (g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of N,. You can leave out the M symbol for molarity. H, NH, initial change equilibrium Continue Submit As O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center 43,203 FEB 19 CH.9 PE ASSESS MacBook Air D00 FI0 吕口 F3 F9 esc F5 F7 F2 ロ口 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY